Quest for the right Drug
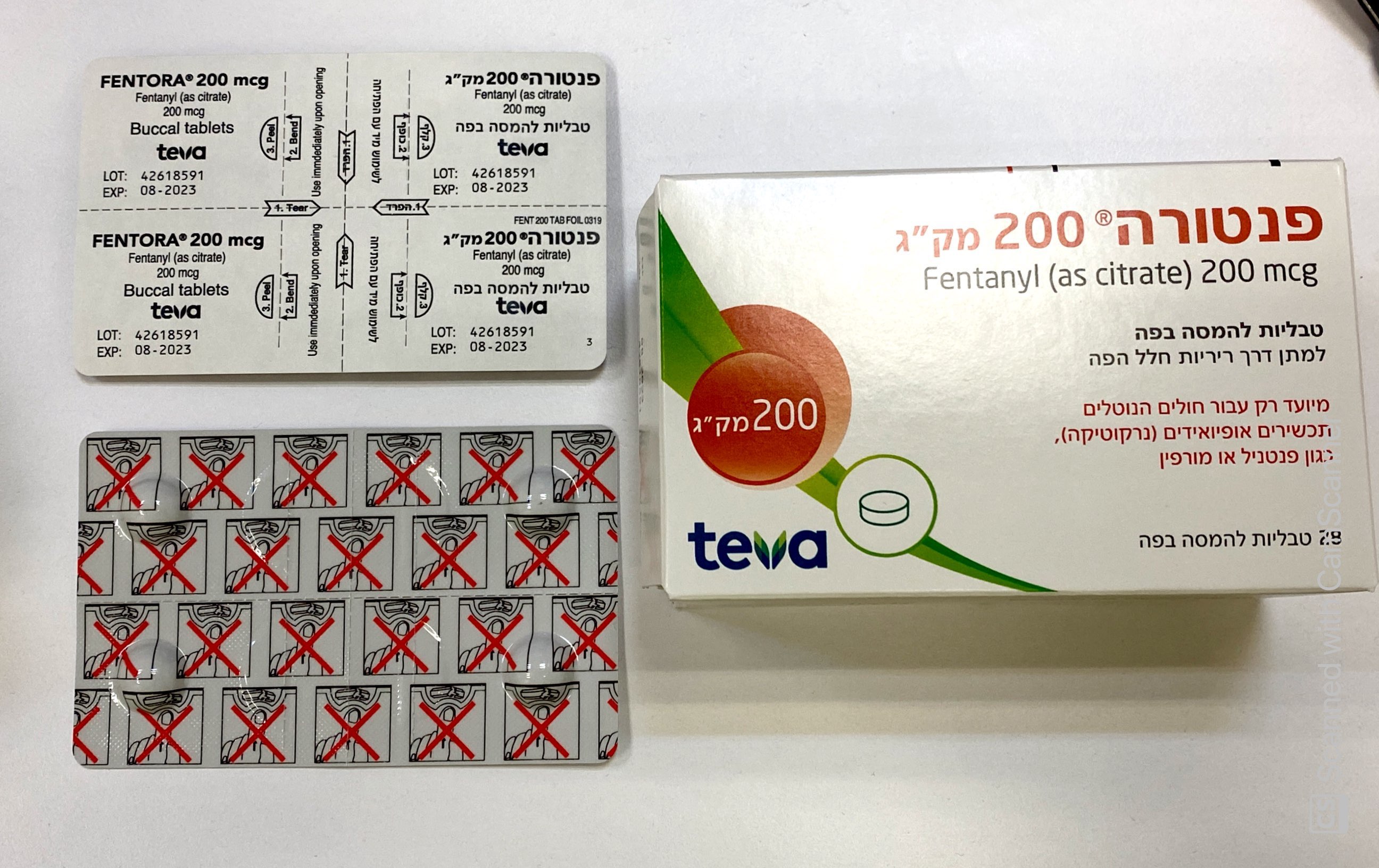
פנטורה ® 200 מק"ג FENTORA ® 200 MCG (FENTANYL AS CITRATE)
תרופה במרשם
תרופה בסל
נרקוטיקה
ציטוטוקסיקה
צורת מתן:
פנים הלחי : BUCCAL
צורת מינון:
טבליה : TABLETS
עלון לרופא
מינוניםPosology התוויות
Indications תופעות לוואי
Adverse reactions התוויות נגד
Contraindications אינטראקציות
Interactions מינון יתר
Overdose הריון/הנקה
Pregnancy & Lactation אוכלוסיות מיוחדות
Special populations תכונות פרמקולוגיות
Pharmacological properties מידע רוקחי
Pharmaceutical particulars אזהרת שימוש
Special Warning עלון לרופא
Physicians Leaflet
Posology : מינונים
4.2 Posology and method of administration Treatment should be initiated by and remain under the guidance of a physician experienced in the management of opioid therapy in cancer patients. Physicians should keep in mind the potential of abuse of fentanyl. Patients should be instructed not to use two different formulations of fentanyl concurrently for the treatment of breakthrough pain, and to dispose of any fentanyl product prescribed for BTP when switching to Fentora. The number of tablet strengths available to the patients at any time should be minimised to prevent confusion and potential overdose. Posology Dose titration Fentora should be individually titrated to an “effective” dose that provides adequate analgesia and minimises adverse reactions. In clinical studies, the effective dose of Fentora for BTP was not predictable from the daily maintenance dose of opioid. Patients should be carefully monitored until an effective dose is reached. Titration in patients not switching from other fentanyl containing products The initial dose of Fentora should be 100 micrograms, titrating upwards as necessary through the range of available tablets strengths (100, 200, 400, 600, 800 micrograms). Titration in patients switching from other fentanyl containing products Due to different absorption profiles, switching must not be done at a 1:1 ratio. If switching from another oral fentanyl citrate product, independent dose titration with Fentora is required as bioavailability between products differs significantly. However, in these patients, a starting dose higher than 100 micrograms may be considered. Method of titration During titration, if adequate analgesia is not obtained within 30 minutes after the start of administration of a single tablet, a second Fentora tablet of the same strength may be used. If treatment of a BTP episode requires more than one tablet, an increase in dose to the next higher available strength should be considered to treat the next BTP episode. During titration, multiple tablets may be used: up to four 100 micrograms or up to four 200 micrograms tablets may be used to treat a single episode of BTP during dose titration according to the following schedule: • If the initial 100 micrograms tablet is not efficacious, the patient can be instructed to treat the next episode of BTP with two 100 micrograms tablets. It is recommended that one tablet should be placed in each side of the mouth. If this dose is considered to be the effective dose, treatment of successive episodes of BTP may be continued with a single 200 micrograms tablet of Fentora. • If a single 200 micrograms tablet of Fentora (or two 100 micrograms tablets) is not considered to be efficacious the patient can be instructed to use two 200 micrograms tablets (or four 100 micrograms tablets) to treat the next episode of BTP. It is recommended that two tablets should be placed in each side of the mouth. If this dose is considered to be the effective dose, treatment of successive episodes of BTP may be continued with a single 400 micrograms tablet of Fentora. • For titration to 600 micrograms and 800 micrograms, tablets of 200 micrograms should be used. Doses above 800 micrograms were not evaluated in clinical studies. No more than two tablets should be used to treat any individual BTP episode, except when titrating using up to four tablets as described above. Patients should wait at least 4 hours before treating another BTP episode with Fentora during titration. Maintenance therapy Once an effective dose has been established during titration, patients should continue to take this dose as a single tablet of that given strength. Breakthrough pain episodes may vary in intensity and the required Fentora dose might increase over time due to progression of the underlying cancer disease. In these cases, a second tablet of the same strength may be used. If a second tablet of Fentora was required for several consecutive times, the usual maintenance dose is to be readjusted (see below). Patients should wait at least 4 hours before treating another BTP episode with Fentora during maintenance therapy. Dose readjustment The maintenance dose of Fentora should be increased when a patient requires more than one tablet per BTP episode for several consecutive BTP episodes. For dose-readjustment the same principles apply as outlined for dose titration (see above). Dose readjustment of the background opioid therapy may be required if patients consistently present with more than four BTP episodes per 24 hours. In absence of adequate pain control, the possibility of hyperalgesia, tolerance and progression of underlying disease should be considered (see section 4.4). Discontinuation of therapy Fentora should be discontinued immediately if the patient no longer experiences breakthrough pain episodes. The treatment for the persistent background pain should be kept as prescribed. If discontinuation of all opioid therapy is required, the patient must be closely followed by the doctor in order to manage the risk of abrupt withdrawal effects. Hepatic or renal impairment Fentora should be administered with caution to patients with moderate or severe hepatic or renal impairment (see section 4.4). Patients with xerostomia Patients experiencing xerostomia are advised to drink water to moisten the buccal cavity prior to administration of Fentora. If this recommendation does not result in an appropriate effervescence, then a switch of therapy may be advised. Use in the elderly (older than 65 years) In clinical studies patients older than 65 years tended to titrate to a lower effective dose than younger patients. It is recommended that increased caution should be exercised in titrating the dose of Fentora in elderly patients. Paediatric population The safety and efficacy of Fentora in children aged 0 to 18 years have not been established. No data are available. Method of administration Fentora tablet once exposed to moisture utilises an effervescent reaction to deliver the active substance. Therefore patients should be instructed not to open the blister until ready to place the tablet in the buccal cavity. Opening the blister package Patients should be instructed NOT to attempt to push the tablet through the blister because this could damage the buccal tablet. The correct method of releasing the tablet from the blister is: One of the blister units should be separated from the blister card by tearing it apart at the perforations. The blister unit should then be flexed along the line printed on the backing foil where indicated. The backing foil should be peeled back to expose the tablet. Patients should be instructed not to attempt to crush or split the tablet. The tablet should not be stored once removed from the blister package as the tablet integrity cannot be guaranteed and a risk of accidental exposure to a tablet can occur. Tablet administration Patients should remove the tablet from the blister unit and immediately place the entire Fentora tablet in the buccal cavity (near a molar between the cheek and gum). The Fentora tablet should not be sucked, chewed or swallowed, as this will result in lower plasma concentrations than when taken as directed. Fentora should be placed and retained within the buccal cavity for a period sufficient to allow disintegration of the tablet which usually takes approximately 14-25 minutes. Alternatively, the tablet could be placed sublingually (see section 5.2). After 30 minutes, if remnants from the Fentora tablet remain, they may be swallowed with a glass of water. The length of time that the tablet takes to fully disintegrate following oromucosal administration does not appear to affect early systemic exposure to fentanyl. Patients should not consume any food and drink when a tablet is in the buccal cavity. In case of buccal mucosa irritation, a change in tablet placement within the buccal cavity should be recommended.

פרטי מסגרת הכללה בסל
התרופה תינתן לטיפול בכאב מתפרץ בחולי סרטן. התחלת הטיפול בתרופה יעשה על פי מרשם של רופא מומחה באונקולוגיה, או בהמטואונקולוגיה או בכאב או בנוירולוגיה או בהרדמה
שימוש לפי פנקס קופ''ח כללית 1994
לא צוין
תאריך הכללה מקורי בסל
01/03/2008
הגבלות
תרופה מוגבלת לרישום ע'י רופא מומחה או הגבלה אחרת
מידע נוסף
עלון מידע לצרכן
11.08.22 - עלון לצרכן אנגלית 11.08.22 - עלון לצרכן עברית 11.08.22 - עלון לצרכן ערבית 18.10.22 - עלון לצרכן עברית 24.11.22 - עלון לצרכן אנגלית 24.11.22 - עלון לצרכן עברית 24.11.22 - עלון לצרכן ערבית 15.08.23 - עלון לצרכן אנגלית 15.08.23 - עלון לצרכן 15.08.23 - עלון לצרכן אנגלית 03.08.23 - עלון לצרכן עברית 27.09.23 - עלון לצרכן אנגלית 27.09.23 - עלון לצרכן עברית 27.09.23 - עלון לצרכן ערבית 23.04.24 - עלון לצרכן עברית 12.07.24 - עלון לצרכן אנגלית 12.07.24 - עלון לצרכן עברית 12.07.24 - עלון לצרכן ערבית 13.04.15 - החמרה לעלון 25.05.17 - החמרה לעלון 07.08.18 - החמרה לעלון 27.11.18 - החמרה לעלון 25.11.19 - החמרה לעלון 26.11.20 - החמרה לעלון 26.01.21 - החמרה לעלון 05.04.21 - החמרה לעלון 05.01.22 - החמרה לעלון 18.10.22 - החמרה לעלון 23.04.24 - החמרה לעלוןלתרופה במאגר משרד הבריאות
פנטורה ® 200 מק"ג