Quest for the right Drug
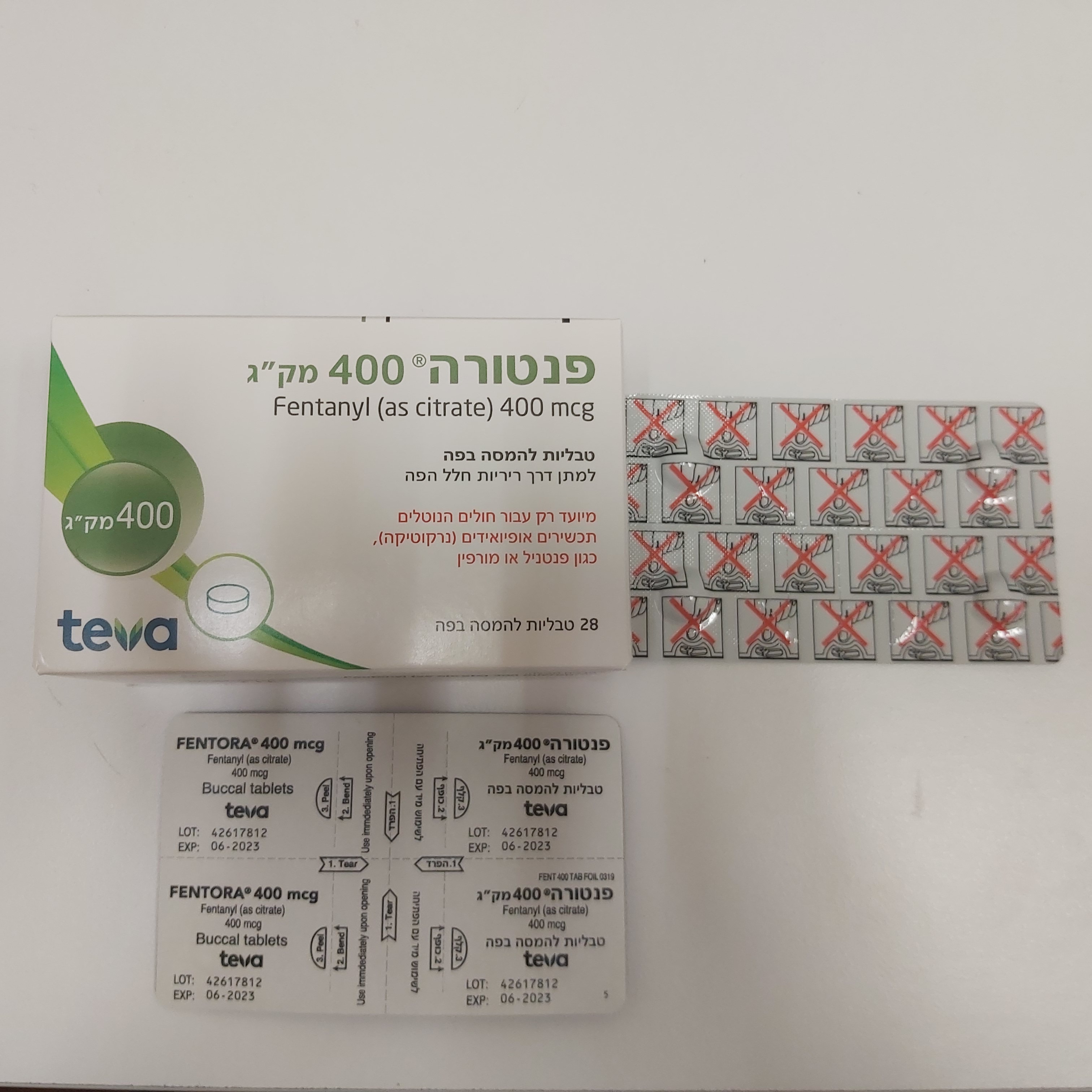
פנטורה ® 400 מק"ג FENTORA ® 400 MCG (FENTANYL AS CITRATE)
תרופה במרשם
תרופה בסל
נרקוטיקה
ציטוטוקסיקה
צורת מתן:
פנים הלחי : BUCCAL
צורת מינון:
טבליה : TABLETS
עלון לרופא
מינוניםPosology התוויות
Indications תופעות לוואי
Adverse reactions התוויות נגד
Contraindications אינטראקציות
Interactions מינון יתר
Overdose הריון/הנקה
Pregnancy & Lactation אוכלוסיות מיוחדות
Special populations תכונות פרמקולוגיות
Pharmacological properties מידע רוקחי
Pharmaceutical particulars אזהרת שימוש
Special Warning עלון לרופא
Physicians Leaflet
Adverse reactions : תופעות לוואי
4.8 Undesirable effects Summary of the safety profile Typical opioid adverse reactions are to be expected with Fentora. Frequently, these will cease or decrease in intensity with continued use of the medicinal product, as the patient is titrated to the most appropriate dose. However, the most serious adverse reactions are respiratory depression (potentially leading to apnoea or respiratory arrest), circulatory depression, hypotension and shock and all patients should be closely monitored for these. The clinical studies of Fentora were designed to evaluate safety and efficacy in treating BTP and all patients were also taking concomitant opioids, such as sustained-release morphine or transdermal fentanyl, for their persistent pain. Therefore it is not possible to definitively separate the effects of Fentora alone. Tabulated list of adverse reactions The following adverse reactions have been reported with Fentora and/or other fentanyl- containing compounds during clinical studies and post marketing experience. Adverse reactions are listed below as MedDRA preferred term by system organ class and frequency (frequencies are defined as: very common (≥1/10), common (≥1/100 to <1/10), uncommon (≥ 1/1,000 to <1/100), rare (1/10,000 to <1/1,000), not known (cannot be estimated from the available data); within each frequency group, undesirable effects are presented in order of decreasing seriousness: Very common Common Uncommon Rare Not known Infections and Oral Pharyngitis Oral pustule infestations candidiasis Blood and Anaemia Thrombocyto- lymphatic Neutropenia penia system disorders Immune Hyper- system senstivity* disorders Endocrine Hypogonadis Adrenal disorders m insufficiency , Androgen deficiency Metabolism Anorexia and nutrition disorders Psychiatric Depression Euphoric mood Drug disorders Anxiety Nervousness dependence Confusional Hallucination (addiction)* state Visual Drug abuse Insomnia hallucination (see sec. 4.4) Mental status Delirium changes Disorientation Very common Common Uncommon Rare Not known Nervous Dizziness Dysgeusia Depressed Cognitive Loss of system Headache Somnolence level of disorder consciousness disorders Lethargy consciousness Motor * Tremor Disturbance in dysfunction Convulsion Sedation attention Hypoaesthesia Balance Migraine disorder Dysarthria Eye Visual Abnormal disorders disturbance sensation in Ocular eye hyperaemia Photopsia Blurred vision Visual acuity reduced Ear and Vertigo labyrinth Tinnitus disorders Ear discomfort Cardiac Tachycardia Bradycardia disorders Vascular Hypotension Flushing disorders Hypertension Hot flush Respiratory, Dyspnoea Respiratory Respiratory thoracic and Pharyngolaryn- depression arrest* mediastinal geal pain Sleep apnoea disorders syndrome Gastro- Nausea Constipation Ileus Oral mucosal intestinal Vomiting Stomatitis Mouth blistering disorders Dry mouth ulceration Dry lip Diarrhoea Oral Abdominal hypoaesthesia pain Oral Gastro- discomfort oesophageal Oral mucosal reflux disease discolouration Stomach Oral soft tissue discomfort disorder Dyspepsia Glossodynia Toothache Tongue blistering Gingival pain Tongue ulceration Tongue disorder Oesophagitis Chapped lips Tooth disorder Hepatobiliary Biliary disorders dilatation Skin and Pruritus Cold sweat Onychorrhexi subcutaneous Hyperhidrosis Facial swelling s tissue Rash Generalised disorders pruritus Alopecia Very common Common Uncommon Rare Not known Musculoskel Myalgia Muscle etal and Back pain twitching connective Muscular tissue weakness disorders Renal and Urinary urinary retention disorders General Application site Peripheral Malaise Pyrexia disorders and reactions oedema Sluggishness Neonatal administration including Fatigue Chest withdrawal site conditions bleeding, pain, Asthenia discomfort syndrome (see ulcer, irritation, Drug Feeling section 4.6) paraesthesia, withdrawal abnormal Drug anaesthesia, syndrome* Feeling jittery tolerance erythema, Chills Thirst oedema, Feeling cold swelling and Feeling hot vesicles Investigations Weight Platelet count decreased decreased Heart rate increased Haematocrit decreased Haemoglobin decreased Injury, Fall poisoning and procedural complications * See section Description of selected adverse reactions Description of selected adverse reactions Tolerance Tolerance can develop on repeated use. Drug dependence Repeated use of Fentora lead to drug dependence, even at therapeutic doses. The risk of drug dependence may vary depending on a patient's individual risk factors, dosage, and duration of opioid treatment (see section 4.4). Opioid withdrawal symptoms such as nausea, vomiting, diarrhoea, anxiety, chills, tremor and sweating have been observed with transmucosal fentanyl. Loss of consciousness and respiratory arrest have been observed in the context of overdose (see section 4.9). Hypersensitivity reactions have been reported in post-marketing experience, including rash, erythema, lip and face swelling, and urticaria (see section 4.4). Reporting of suspected adverse reactions Reporting suspected adverse reactions after authorisation of the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the medicinal product. Any suspected adverse events should be reported to the Ministry of Health according to the National Regulation by using an online form https://sideeffects.health.gov.il

פרטי מסגרת הכללה בסל
התרופה תינתן לטיפול בכאב מתפרץ בחולי סרטן. התחלת הטיפול בתרופה יעשה על פי מרשם של רופא מומחה באונקולוגיה, או בהמטואונקולוגיה או בכאב או בנוירולוגיה או בהרדמה
שימוש לפי פנקס קופ''ח כללית 1994
לא צוין
תאריך הכללה מקורי בסל
01/03/2008
הגבלות
תרופה מוגבלת לרישום ע'י רופא מומחה או הגבלה אחרת
מידע נוסף
עלון מידע לצרכן
11.08.22 - עלון לצרכן אנגלית 11.08.22 - עלון לצרכן עברית 11.08.22 - עלון לצרכן ערבית 18.10.22 - עלון לצרכן עברית 24.11.22 - עלון לצרכן אנגלית 24.11.22 - עלון לצרכן עברית 24.11.22 - עלון לצרכן ערבית 15.08.23 - עלון לצרכן אנגלית 15.08.23 - עלון לצרכן 15.08.23 - עלון לצרכן אנגלית 03.08.23 - עלון לצרכן עברית 27.09.23 - עלון לצרכן אנגלית 27.09.23 - עלון לצרכן עברית 27.09.23 - עלון לצרכן ערבית 23.04.24 - עלון לצרכן עברית 12.07.24 - עלון לצרכן אנגלית 12.07.24 - עלון לצרכן עברית 12.07.24 - עלון לצרכן ערבית 13.04.15 - החמרה לעלון 25.05.17 - החמרה לעלון 07.08.18 - החמרה לעלון 27.11.18 - החמרה לעלון 25.11.19 - החמרה לעלון 26.11.20 - החמרה לעלון 26.01.21 - החמרה לעלון 05.04.21 - החמרה לעלון 05.01.22 - החמרה לעלון 18.10.22 - החמרה לעלון 23.04.24 - החמרה לעלוןלתרופה במאגר משרד הבריאות
פנטורה ® 400 מק"ג