Quest for the right Drug
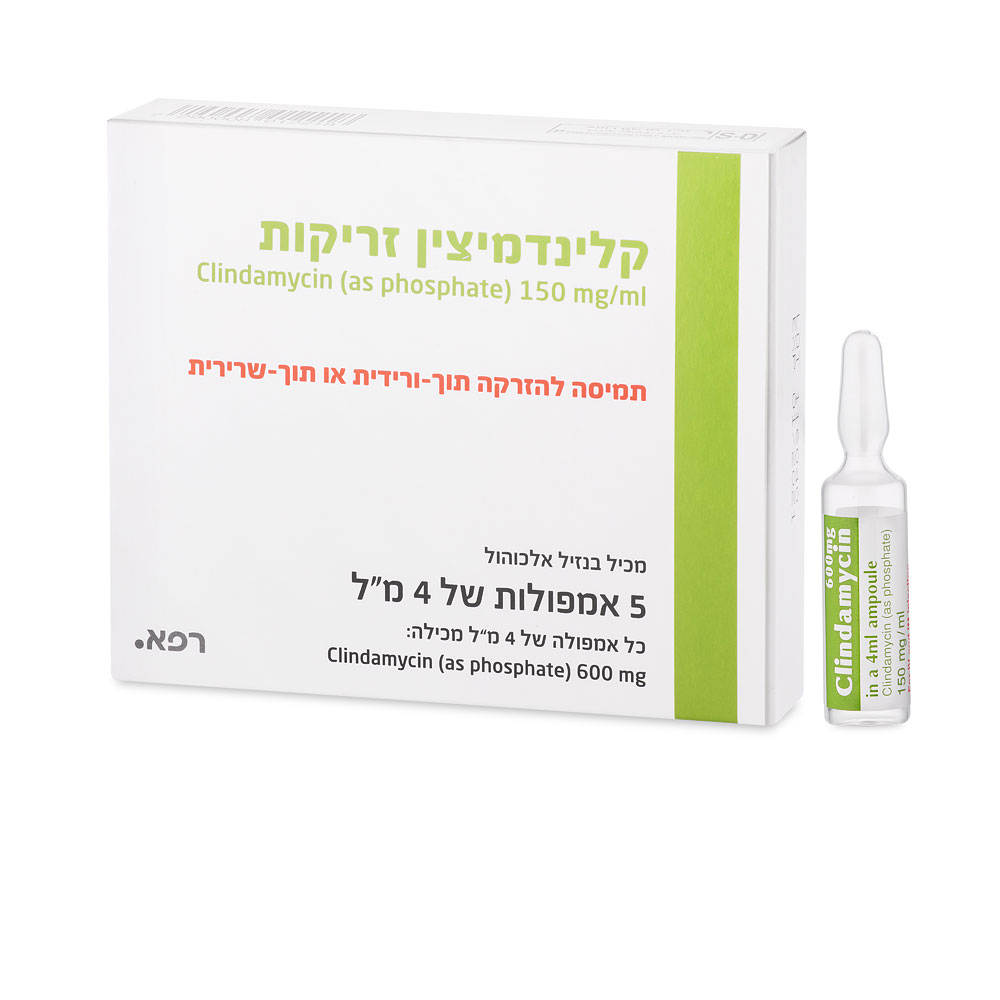
קלינדמיצין זריקות CLINDAMYCIN INJECTIONS (CLINDAMYCIN AS PHOSPHATE)
תרופה במרשם
תרופה בסל
נרקוטיקה
ציטוטוקסיקה
צורת מתן:
תוך-שרירי, תוך-ורידי : I.M, I.V
צורת מינון:
תמיסה להזרקה : SOLUTION FOR INJECTION
עלון לרופא
מינוניםPosology התוויות
Indications תופעות לוואי
Adverse reactions התוויות נגד
Contraindications אינטראקציות
Interactions מינון יתר
Overdose הריון/הנקה
Pregnancy & Lactation אוכלוסיות מיוחדות
Special populations תכונות פרמקולוגיות
Pharmacological properties מידע רוקחי
Pharmaceutical particulars אזהרת שימוש
Special Warning עלון לרופא
Physicians Leaflet
Pharmaceutical particulars : מידע רוקחי
6. Pharmaceutical particulars Clindamycin ampoules are not intended for multiple dosing and any unused portion should be discarded. Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit. No ampoule file is needed to open the ampoules. The neck of the ampoule is pre-scored at the point of constriction. A dot on the ampoule head helps to orientate the ampoule. Take the ampoule and face the dot. The ampoule opens easily by placing the thumb on the dot and gently pressing downwards. 6.1 List of excipients Benzyl alcohol, disodium edetate, water for injection, sodium hydroxide (for pH adjustment), hydrochloric acid (for pH adjustment). 6.2 Incompatibilities Solutions of clindamycin salts have a low pH and incompatibilities may reasonably be expected with alkaline preparations or drugs unstable at low pH. Incompatibility has been reported with: ampicillin sodium, aminophylline, barbiturates, calcium gluconate, ceftriaxone sodium, ciprofloxacin, diphenylhydantoin, idarubicin hydrochloride, magnesium sulphate, phenytoin sodium and ranitidine hydrochloride. 6.3 Shelf life The expiry date of the product is indicated on the packaging materials. 6.4 Special precautions for storage Store below 25°C. Do not freeze. Crystallization may occur if stored at low temperature; the crystals resolubilize on warming to room temperature (25ºC), but care should be exercised to ensure that all crystals have redissolved. 6.5 Nature and contents of container Clear glass 5 ml ampoule made of borosilicate glass type 1. Each ampoule contains 4 ml solution. 6.6 Special precautions for disposal and other handling A similar clindamycin product has been shown to be physically and chemically compatible for at least 24 hours in dextrose 5% water and sodium chloride injection solutions containing the following antibiotics in usually administered concentrations: Amikacin sulphate, aztreonam, cefamandole nafate, cephazolin sodium, cefotaxime sodium, cefoxitin sodium, ceftazidime sodium, ceftizoxime sodium, gentamicin sulphate, netilmicin sulphate, piperacillin and tobramycin. The compatibility and duration of stability of drug admixtures will vary depending upon concentration and other conditions. 7. Registration holder: Rafa Laboratories Ltd., P.O.Box 405, Jerusalem 9100301

שימוש לפי פנקס קופ''ח כללית 1994
התרופה תימצא רק בבתי חולים ותנופק לחולים אמבולטורים רק באמצעותם
תאריך הכללה מקורי בסל
01/01/1995
הגבלות
תרופה אשפוזית לפי החלטת משרד הבריאות
מידע נוסף
