Quest for the right Drug
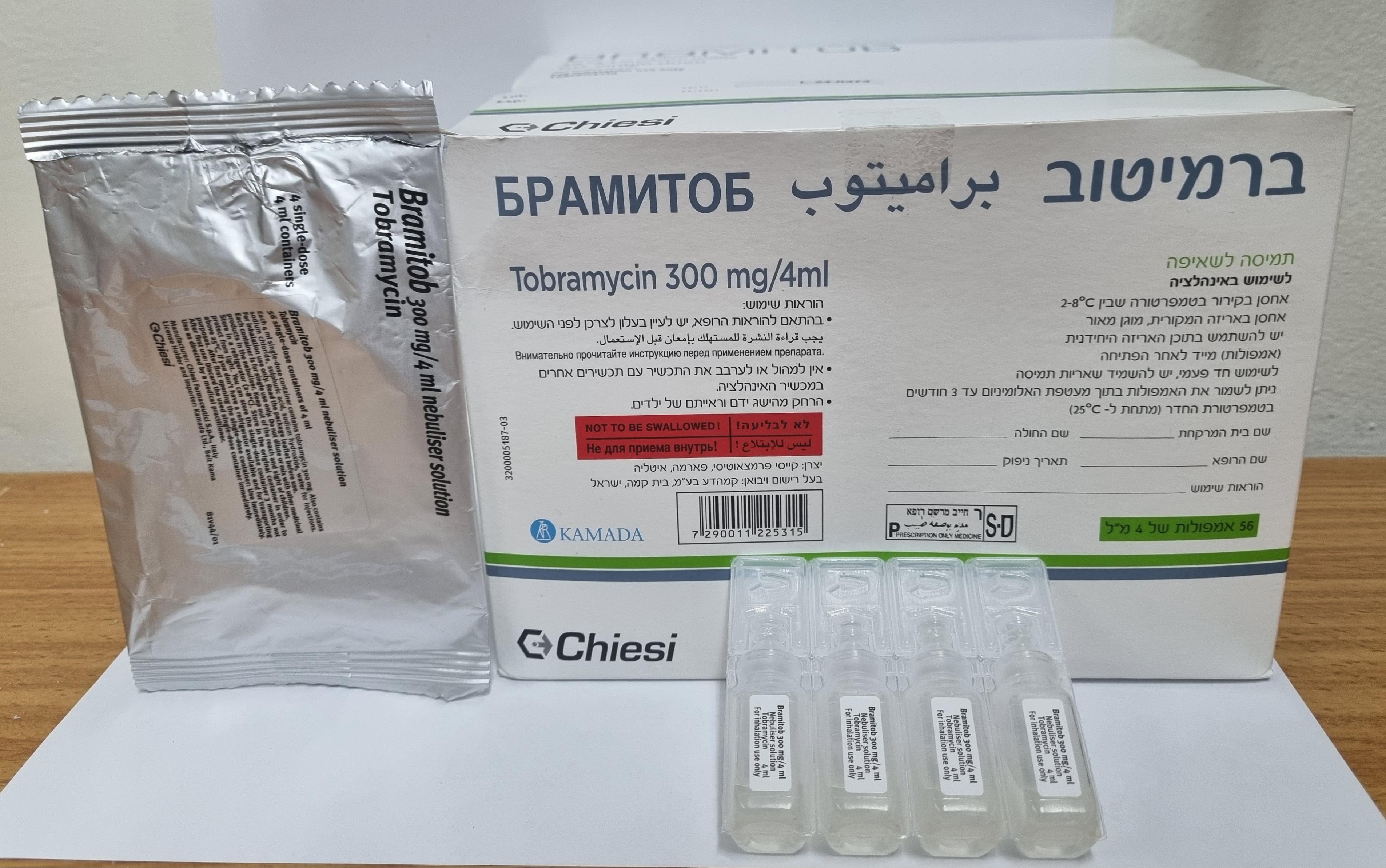
ברמיטוב BRAMITOB (TOBRAMYCIN)
תרופה במרשם
תרופה בסל
נרקוטיקה
ציטוטוקסיקה
צורת מתן:
ערפול : NEBULISATION
צורת מינון:
תמיסה לשאיפה : SOLUTION FOR INHALATION
עלון לרופא
מינוניםPosology התוויות
Indications תופעות לוואי
Adverse reactions התוויות נגד
Contraindications אינטראקציות
Interactions מינון יתר
Overdose הריון/הנקה
Pregnancy & Lactation אוכלוסיות מיוחדות
Special populations תכונות פרמקולוגיות
Pharmacological properties מידע רוקחי
Pharmaceutical particulars אזהרת שימוש
Special Warning עלון לרופא
Physicians Leaflet
Posology : מינונים
4.2 Posology and method of administration Bramitob is intended for inhalation only and not for parenteral use. Consideration should be given to official guidance on the appropriate use of antibacterial agents. Therapy should be initiated by a physician experienced in the management of cystic fibrosis. The recommended dose for adults and children above 6 years is one single-dose container (300mg) twice daily (morning and evening) for 28 days. The dose interval should be as close as possible to 12 hours. After 28 days of therapy with Bramitob, patients should stop treatment for the next 28 days. Alternate cycles of 28-days of active therapy followed by 28 days without treatment should be maintained (a cycle of 28 days with therapy and 28 days without treatment). Children under 6 years old The efficacy and safety of Bramitob have not been demonstrated in patients less than 6 years of age. Elderly patients Tobramycin should be used with caution in elderly patients who may have reduced renal function (see section 4.4). Patients with renal impairment Tobramycin should be used with caution in patients with known or suspected renal dysfunction. Bramitob should be discontinued in the case of nephrotoxicity until serum concentration of tobramycin falls below 2 µg/mL (see section 4.4). Patients with hepatic insufficiency No changes in Bramitob dose are required in hepatic insufficiency. Dosage is not adjusted for body weight. All patients should be administered one single-dose container of Bramitob (300 mg of tobramycin) twice daily. Treatment with tobramycin should be continued on a cyclical basis for as long as the physician considers the patient is gaining clinical benefit from the inclusion of Bramitob in their treatment regimen. If clinical deterioration of pulmonary status is evident, additional anti-pseudomonal therapy should be considered. Method of Administration: The single-dose container should be opened just before use. Any unused solution that is not immediately used should be discarded and not stored for re-use. Administration of Bramitob should be carried out following general hygienic standards. The apparatus used should be clean and working correctly; the nebuliser, that should be for personal use only, should be kept clean and regularly disinfected. For cleaning and disinfection of the nebuliser, refer to the instructions provided with the nebuliser. Maximum tolerated daily dose The maximum tolerated daily dose of Bramitob has not been established. Instructions for opening the container: 1) Bend the single-dose container in both directions 2) Detach the single-dose container from the strip, firstly above then in the middle 3) Open the single-dose container by rotating the flap as indicated by the arrow 4) Exerting a moderate pressure on the single-dose container’s walls, let the medicinal product flow into the glass tube of the nebuliser. The contents of one single-dose container (300mg) emptied into the nebuliser, should be administered by inhalation over approximately a 15-minute period using a PARI LC PLUS reusable nebuliser equipped with PARI TURBO BOY compressor (drug delivery rate 8 mg/min, total drug delivery 80 mg, mass median aerodynamic diameter: D10 1.1µm, D50 5.9µm, D90 13.3µm). Bramitob is inhaled while the patient is sitting or standing upright and breathing normally through the mouthpiece of the nebuliser. Nose clips may help the patient with breathing through the mouth. The patient should continue their standard regimen of chest physiotherapy. The use of appropriate bronchodilators should continue as thought clinically necessary. In patients receiving several different respiratory therapies, it is recommended that they are taken in the following order: bronchodilator, respiratory physiotherapy, other inhaled medicinal products, and finally Bramitob. Bramitob should not be mixed with other inhalation medicinal products.

פרטי מסגרת הכללה בסל
התרופה האמורה תינתן לטיפול נגד פסאודומונס ארוגינוזה בחולי לייפת כיסתית (Cystic fibrosis)
מסגרת הכללה בסל
התוויות הכלולות במסגרת הסל
| התוויה | תאריך הכללה | תחום קליני | Class Effect | מצב מחלה |
|---|---|---|---|---|
| התרופה האמורה תינתן לטיפול נגד פסאודומונס ארוגינוזה בחולי לייפת כיסתית (Cystic fibrosis) | 15/05/2006 |
שימוש לפי פנקס קופ''ח כללית 1994
לא צוין
תאריך הכללה מקורי בסל
15/05/2006
הגבלות
תרופה מוגבלת לרישום ע'י רופא מומחה או הגבלה אחרת
מידע נוסף
