Quest for the right Drug
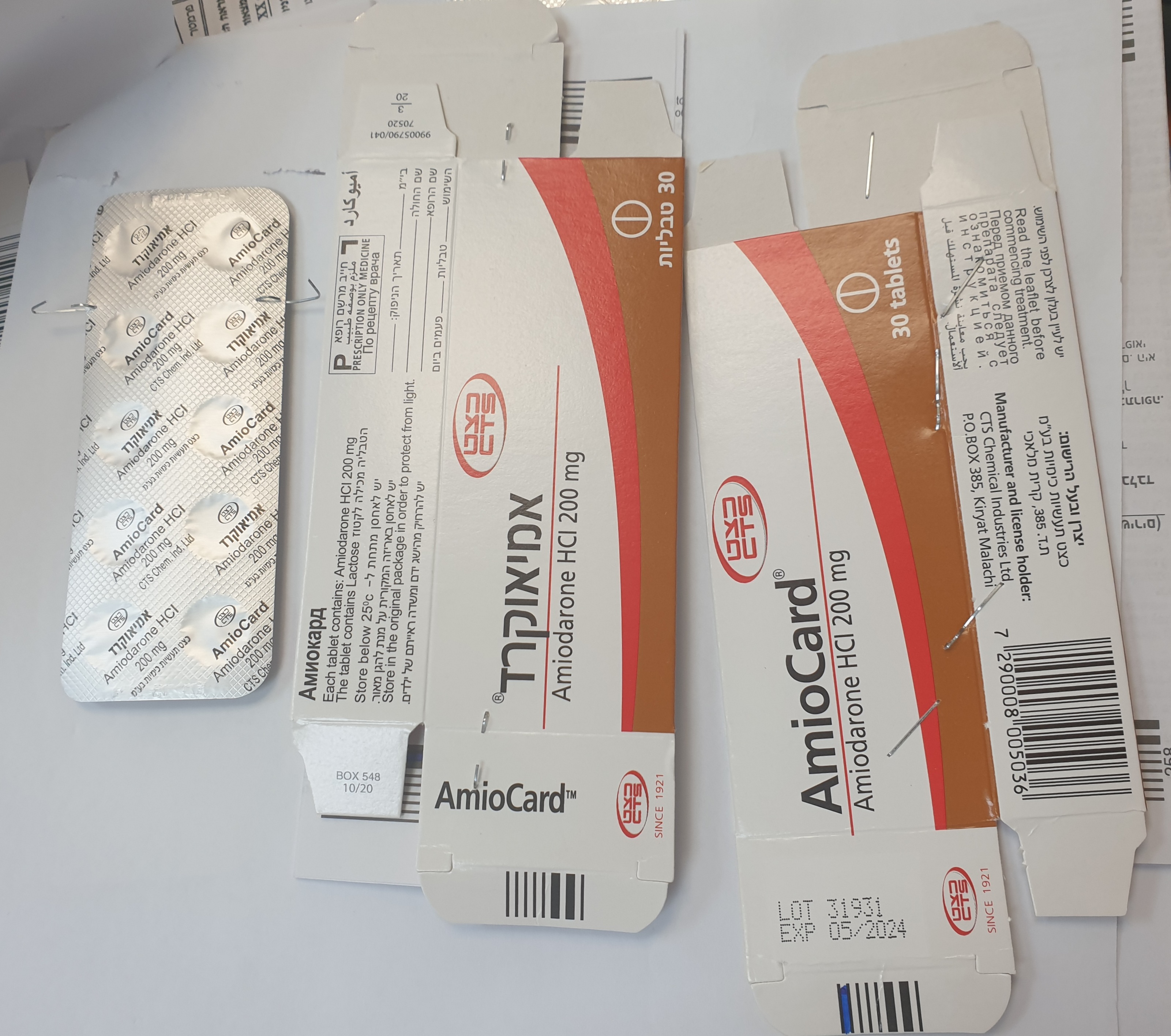
אמיאוקרד AMIOCARD (AMIODARONE HYDROCHLORIDE)
תרופה במרשם
תרופה בסל
נרקוטיקה
ציטוטוקסיקה
צורת מתן:
פומי : PER OS
צורת מינון:
טבליה : TABLETS
עלון לרופא
מינוניםPosology התוויות
Indications תופעות לוואי
Adverse reactions התוויות נגד
Contraindications אינטראקציות
Interactions מינון יתר
Overdose הריון/הנקה
Pregnancy & Lactation אוכלוסיות מיוחדות
Special populations תכונות פרמקולוגיות
Pharmacological properties מידע רוקחי
Pharmaceutical particulars אזהרת שימוש
Special Warning עלון לרופא
Physicians Leaflet
Pharmacological properties : תכונות פרמקולוגיות
Pharmacodynamic Properties
5.1 Pharmacodynamic properties Amiodarone hydrochloride is an antiarrhythmic. No controlled studies have been undertaken. In published studies the safety of amiodarone was evaluated in 1118 paediatric patients with various arrhythmias. The following doses were used in paediatric clinical trials. Oral - Loading dose: 10 to 20 mg/kg/day for 7 to 10 days (or 500 mg/m²/day ifexpressed per square meter) - Maintenance dose: the minimum effective dosage should be used; according to individual response, it may range between 5 to 10 mg/kg/day (or 250 mg/m²/dayif expressed per square meter) Intravenous - Loading dose: 5 mg/kg body weight over 20 minutes to 2 hours - Maintenance dose: 10 to 15 mg/kg/day from a few hours to several days If needed, oral therapy may be initiated concomitantly at the usual loading dose.
Pharmacokinetic Properties
5.2 Pharmacokinetic properties Amiodarone is strongly protein bound and the plasma half life is usually of the order of 50 days. However there may be considerable inter-patient variation; in individual patients a half life of less than 20 days and a half life of more than 100 days has been reported. High doses of AMIOCARD, for example 600mg/day, should be given initially to achieve effective tissue levels as rapidly as possible. Owing to the long half life of the drug, a maintenance dose of only 200mg/day, or less is usually necessary. Sufficient time must be allowed for a new distribution equilibrium to be achieved between adjustments of dose. The long half life is a valuable safeguard for patients with potentially lethal arrhythmias as omission of occasional doses does not significantly influence the protection afforded by AMIOCARD. No controlled paediatric studies have been undertaken. In the limited published dateavailable in paediatric patients, there were no differences noted compared to adults. Amiodarone is metabolised mainly by CYP3A4, and also by CYP2C8. Amiodaroneand its metabolite, desethylamiodarone, exhibit a potential in vitro to inhibit CYP1A1, CYP1A2, CYP2C9, CYP2C19, CYP2D6, CYP3A4, CYP2A6, CYP2B6 and 2C8. Amiodarone and desethylamiodarone have also a potential to inhibit some transporters such as P-gp and organic cation transporter (OCT2) (One study shows a1.1% increase in concentration of creatine (a OCT 2 substrate). In vivo data describeamiodarone interactions on CYP3A4, CYP2C9, CYP2D6 and P-gp substrates.

שימוש לפי פנקס קופ''ח כללית 1994
Ventricular & supraventricular arrhythmias not responding to other treatments, tachyarrhythmias associated with Wolff-Parkinson-White syndrome
תאריך הכללה מקורי בסל
01/01/1995
הגבלות
תרופה שאושרה לשימוש כללי בקופ'ח
מידע נוסף
עלון מידע לצרכן
01.08.21 - עלון לצרכן אנגלית 01.08.21 - עלון לצרכן עברית 01.08.21 - עלון לצרכן ערבית 11.10.22 - עלון לצרכן אנגלית 11.10.22 - עלון לצרכן עברית 11.10.22 - עלון לצרכן ערבית 12.06.23 - עלון לצרכן אנגלית 12.06.23 - עלון לצרכן עברית 12.06.23 - עלון לצרכן ערבית 13.05.12 - החמרה לעלון 19.10.15 - החמרה לעלון 01.08.21 - החמרה לעלון 11.10.22 - החמרה לעלוןלתרופה במאגר משרד הבריאות
אמיאוקרד