Quest for the right Drug
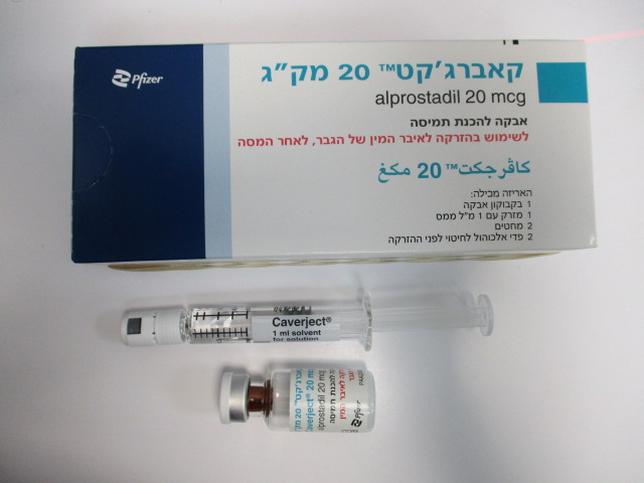
קאברג'קט 20 מק"ג CAVERJECT 20 MCG (ALPROSTADIL)
תרופה במרשם
תרופה בסל
נרקוטיקה
ציטוטוקסיקה
צורת מתן:
תוך-פיני : INTRACAVERNOSAL
צורת מינון:
אבקה להכנת תמיסה לזריקה : POWDER FOR SOLUTION FOR INJECTION
עלון לרופא
מינוניםPosology התוויות
Indications תופעות לוואי
Adverse reactions התוויות נגד
Contraindications אינטראקציות
Interactions מינון יתר
Overdose הריון/הנקה
Pregnancy & Lactation אוכלוסיות מיוחדות
Special populations תכונות פרמקולוגיות
Pharmacological properties מידע רוקחי
Pharmaceutical particulars אזהרת שימוש
Special Warning עלון לרופא
Physicians Leaflet
Pharmacological properties : תכונות פרמקולוגיות
Pharmacodynamic Properties
5.1 Pharmacodynamic properties Pharmacotherapeutic group: Drugs used in erectile dysfunction ATC code: G04B E01 Alprostadil is present in various mammalian tissues and fluids. It has a diverse pharmacologic profile, among which some of its more important effects are vasodilation, inhibition of platelet aggregation, inhibition of gastric secretion, and stimulation of intestinal and uterine smooth muscle. The pharmacologic effect of alprostadil in the treatment of erectile dysfunction is presumed to be mediated by inhibition of alpha1-adrenergic activity in penile tissue and by its relaxing effect on cavernosal smooth muscle.
Pharmacokinetic Properties
5.2 Pharmacokinetic properties Following intracavernous injection of 20 micrograms of alprostadil, mean peripheral levels of alprostadil at 30 and 60 minutes after injection are not significantly greater than baseline levels of endogenous PGE1. Peripheral levels of the major circulating metabolite, 15-oxo-13,14-dihydro-PGE1, increase to reach a peak 30 minutes after injection and return to pre-dose levels by 60 minutes after injection. Any alprostadil entering the systemic circulation from the corpus cavernosum will be rapidly metabolised. Following intravenous administration, approximately 80% of the circulating alprostadil is metabolised in one pass through the lungs, primarily by beta- and omega-oxidation. The metabolites are excreted primarily by the kidney and excretion is essentially complete within 24 hours. There is no evidence of tissue retention of alprostadil or its metabolites following intravenous administration.

שימוש לפי פנקס קופ''ח כללית 1994
לא צוין
תאריך הכללה מקורי בסל
לא צוין
הגבלות
לא צוין
מידע נוסף
