Quest for the right Drug
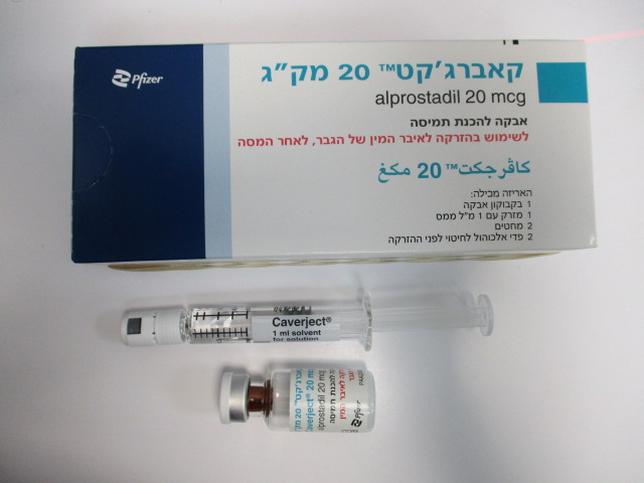
קאברג'קט 20 מק"ג CAVERJECT 20 MCG (ALPROSTADIL)
תרופה במרשם
תרופה בסל
נרקוטיקה
ציטוטוקסיקה
צורת מתן:
תוך-פיני : INTRACAVERNOSAL
צורת מינון:
אבקה להכנת תמיסה לזריקה : POWDER FOR SOLUTION FOR INJECTION
עלון לרופא
מינוניםPosology התוויות
Indications תופעות לוואי
Adverse reactions התוויות נגד
Contraindications אינטראקציות
Interactions מינון יתר
Overdose הריון/הנקה
Pregnancy & Lactation אוכלוסיות מיוחדות
Special populations תכונות פרמקולוגיות
Pharmacological properties מידע רוקחי
Pharmaceutical particulars אזהרת שימוש
Special Warning עלון לרופא
Physicians Leaflet
Posology : מינונים
4.2 Posology and method of administration Caverject is administered by direct intracavernous injection. A half inch, 27 to 30 gauge needle is generally recommended. The dose of Caverject should be individualised for each patient by careful titration under supervision by a physician. The intracavernosal injection must be done under sterile conditions. The site of injection is usually along the dorsolateral aspect of the proximal third of the penis. Visible veins should be avoided. Both the side of the penis that is injected and the site of injection must be alternated; prior to the injection, the injection site must be cleansed with an alcohol swab. To reconstitute Caverject using the prefilled diluent syringe: flip off the plastic cap from the vial, and use one of the swabs to wipe the rubber cap. Fit the 22 gauge needle to the syringe. Inject the 1 ml of diluent into the vial, and shake to dissolve the powder entirely. Withdraw slightly more than the required dose of Caverject solution, remove the 22 gauge needle, and fit the 30 gauge needle. Adjust volume to the required dose for injection. Following administration, any unused contents of the vial or syringe should be discarded. A. As an aid to aetiologic diagnosis. i) Subjects without evidence of neurological dysfunction; 20 micrograms alprostadil to be injected into the corpus cavernosum and massaged through the penis. Should an ensuing erection persist for more than one hour detumescent therapy (please refer to section 4.9) should be employed prior to the subject leaving the clinic to prevent a risk of priapism. Over 80% of subjects may be expected to respond to a single 20 micrograms dose of alprostadil. At the time of discharge from the clinic, the erection should have subsided entirely and the penis must be in a completely flaccid state. ii) Subjects with evidence of neurological dysfunction; these patients can be expected to respond to lower doses of alprostadil. In subjects with erectile dysfunction caused by neurologic disease/trauma the dose for diagnostic testing must not exceed 10 micrograms and an initial dose of 5 micrograms is likely to be appropriate. Should an ensuing erection persist for more than one hour detumescent therapy (please refer to section 4.9) should be employed prior to the subject leaving the clinic to prevent a risk of priapism. At the time of discharge from the clinic, the erection should have subsided entirely and the penis must be in a completely flaccid state. B. Treatment The initial dose of alprostadil in patients with erectile dysfunction of neurogenic origin secondary to spinal cord injury is 1.25 micrograms, with a second dose of 2.5 micrograms, a third of 5 micrograms, and subsequent incremental increases of 5 micrograms until an optimal dose is achieved. For erectile dysfunction of vasculogenic, psychogenic, or mixed aetiology, the initial dose is 2.5 micrograms. The second dose should be 5 micrograms if there is a partial response, and 7.5 micrograms if there is no response. Subsequent incremental increases of 5-10 micrograms should be given until an optimal dose is achieved. If there is no response to the administered dose, then the next higher dose may be given within 1 hour. If there is a response, there should be at least a 1-day interval before the next dose is given. The usual maximum recommended frequency of injection is no more than once daily and no more than three times weekly. The first injections of alprostadil must be done by medically trained personnel. After proper training and instruction, alprostadil may be injected at home. If self-administration is planned, the physician should make an assessment of the patient's skill and competence with the procedure. It is recommended that patients are regularly monitored (e.g. every 3 months) particularly in the initial stages of self injection therapy when dose adjustments may be needed. The dose that is selected for self-injection treatment should provide the patient with an erection that is satisfactory for sexual intercourse. It is recommended that the dose administered produces a duration of the erection not exceeding one hour. If the duration is longer, the dose should be reduced. The majority of patients achieve a satisfactory response with doses in the range of 5 to 20 micrograms. Doses of greater than 60 micrograms of alprostadil are not recommended. The lowest effective dose should be used. Paediatric population: Caverject is not indicated for paediatric use (see section 4.4 Benzyl alcohol).

שימוש לפי פנקס קופ''ח כללית 1994
לא צוין
תאריך הכללה מקורי בסל
לא צוין
הגבלות
לא צוין
מידע נוסף
