Quest for the right Drug
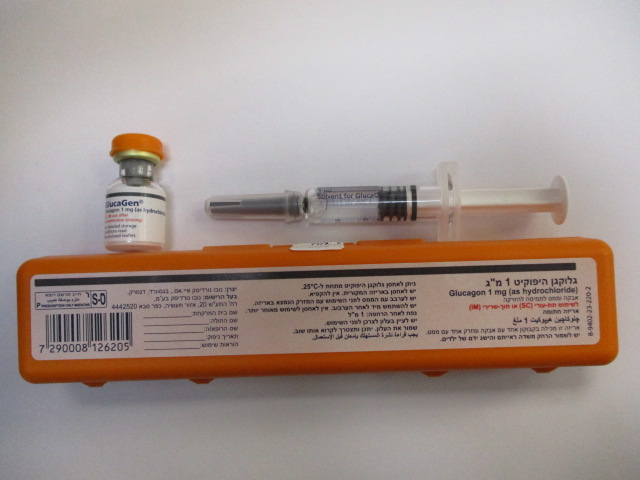
גלוקגן היפוקיט 1 מ"ג GLUCAGEN HYPOKIT 1 MG (GLUCAGON AS HYDROCHLORIDE)
תרופה במרשם
תרופה בסל
נרקוטיקה
ציטוטוקסיקה
צורת מתן:
תת-עורי, תוך-שרירי : S.C, I.M
צורת מינון:
אבקה וממס להכנת תמיסה להזרקה : POWDER AND SOLVENT FOR SOLUTION FOR INJECTION
עלון לרופא
מינוניםPosology התוויות
Indications תופעות לוואי
Adverse reactions התוויות נגד
Contraindications אינטראקציות
Interactions מינון יתר
Overdose הריון/הנקה
Pregnancy & Lactation אוכלוסיות מיוחדות
Special populations תכונות פרמקולוגיות
Pharmacological properties מידע רוקחי
Pharmaceutical particulars אזהרת שימוש
Special Warning עלון לרופא
Physicians Leaflet
Adverse reactions : תופעות לוואי
4.8 Undesirable effects Summary of the safety profile Severe adverse reactions are very rare, although nausea, vomiting and abdominal pain may occur occasionally. Hypersensitivity reactions, including anaphylactic reactions, have been reported as ‘very rare’ (less than 1 case per 10,000 patients). Tabulated summary of adverse reactions Frequencies of undesirable effects considered related to treatment with GlucaGen during clinical trials and/or post-marketing surveillance are presented below. Undesirable effects which have not been observed in clinical trials, but have been reported spontaneously, are presented as ‘very rare’. During marketed use reporting of adverse drug reactions is very rare (< 1/10,000). However, post-marketing experience is subject to under-reporting and this reporting rate should be interpreted in that light. System Organ Class Subject incidence Adverse drug reaction Immune system Very rare < 1/10,000 Hypersensitivity reactions disorders including anaphylactic reaction/shock Gastrointestinal Common ≥ 1/100 to < 1/10 Nausea disorders Uncommon ≥ 1/1,000 to < 1/100 Vomiting Rare ≥ 1/10,000 to < 1/1,000 Abdominal pain General disorders and Not known (cannot be estimated from the Injection site reactions administration site available data) conditions Paediatric population Based on data from clinical trials and post-marketing experience, the frequency, type and severity of adverse reactions observed in children are expected to be the same as in adults. GlucaGen HypoKit IL SPC MAR 2023-Notification Other special populations Based on data from clinical trials and post-marketing experience, the frequency, type and severity of adverse reactions observed in elderly patients and in patients with renal or hepatic impairment are expected to be the same as in the general population. Reporting of suspected adverse reactions Reporting suspected adverse reactions after authorisation of the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the medicinal product. Any suspected adverse events should be reported to the Ministry of Health according to the National Regulation by using an online form: https://sideeffects.health.gov

שימוש לפי פנקס קופ''ח כללית 1994
Acute hypoglycemia (only if liver glycogen is available)
תאריך הכללה מקורי בסל
01/01/1995
הגבלות
תרופה שאושרה לשימוש כללי בקופ'ח
מידע נוסף
