Quest for the right Drug
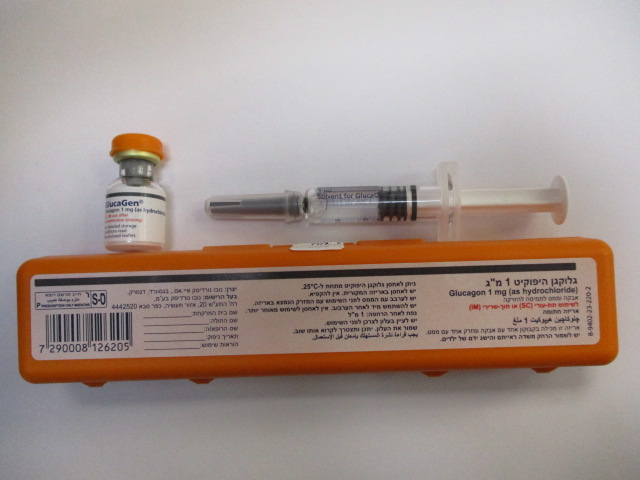
גלוקגן היפוקיט 1 מ"ג GLUCAGEN HYPOKIT 1 MG (GLUCAGON AS HYDROCHLORIDE)
תרופה במרשם
תרופה בסל
נרקוטיקה
ציטוטוקסיקה
צורת מתן:
תת-עורי, תוך-שרירי : S.C, I.M
צורת מינון:
אבקה וממס להכנת תמיסה להזרקה : POWDER AND SOLVENT FOR SOLUTION FOR INJECTION
עלון לרופא
מינוניםPosology התוויות
Indications תופעות לוואי
Adverse reactions התוויות נגד
Contraindications אינטראקציות
Interactions מינון יתר
Overdose הריון/הנקה
Pregnancy & Lactation אוכלוסיות מיוחדות
Special populations תכונות פרמקולוגיות
Pharmacological properties מידע רוקחי
Pharmaceutical particulars אזהרת שימוש
Special Warning עלון לרופא
Physicians Leaflet
Special Warning : אזהרת שימוש
4.4 Special warnings and precautions for use Traceability In order to improve the traceability of biological medicinal products, the name of the administered product should be clearly recorded. It is reccomended to record the batch umber as well. Due to the instability of GlucaGen in solution, the product should be given immediately after reconstitution. To prevent relapse of the hypoglycaemia, oral carbohydrates should be given to restore the liver glycogen, when the patient has responded to the treatment. Glucagon will not be effective in patients whose liver glycogen is depleted. For that reason, glucagon has little or no effect when the patient has been fasting for a prolonged period, or is suffering from adrenal insufficiency, chronic hypoglycaemia or alcohol induced hypoglycaemia. Glucagon, unlike adrenaline, has no effect upon muscle phosphorylase and therefore cannot assist in the transference of carbohydrate from the much larger stores of glycogen that are present in the skeletal muscle. Glucagon reacts antagonistically towards insulin and caution should be observed if GlucaGen is used in patients with insulinoma. Glucagon stimulates the release of catecholamines. In the presence of phaeocromocytoma, glucagon can cause the tumour to release large amounts of catecholamines, which will cause an acute hypertensive reaction. Glucagon is contraindicated in patients with phaeochromocytoma (see section 4.3). Excipients GlucaGen contains less than 1 mmol sodium (23 mg) per maximum dose (2 ml), that is to say essentially ‘sodium-free’.
Effects on Driving
4.7 Effects on ability to drive and use machines After a severe hypoglycaemic event, the patient’s ability to concentrate and react may be impaired. Therefore the patient should not drive or operate machinery after a severe hypoglycaemic event until the patient has stabilised.

שימוש לפי פנקס קופ''ח כללית 1994
Acute hypoglycemia (only if liver glycogen is available)
תאריך הכללה מקורי בסל
01/01/1995
הגבלות
תרופה שאושרה לשימוש כללי בקופ'ח
מידע נוסף
