Quest for the right Drug
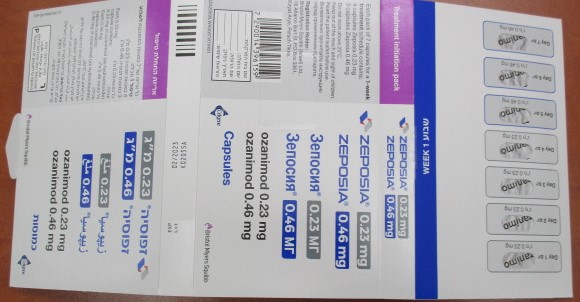
זפוסיה 0.46 מ"ג ZEPOSIA 0.46 MG (OZANIMOD AS HYDROCHLORIDE)
תרופה במרשם
תרופה בסל
נרקוטיקה
ציטוטוקסיקה
צורת מתן:
פומי : PER OS
צורת מינון:
קפסולות : CAPSULES
עלון לרופא
מינוניםPosology התוויות
Indications תופעות לוואי
Adverse reactions התוויות נגד
Contraindications אינטראקציות
Interactions מינון יתר
Overdose הריון/הנקה
Pregnancy & Lactation אוכלוסיות מיוחדות
Special populations תכונות פרמקולוגיות
Pharmacological properties מידע רוקחי
Pharmaceutical particulars אזהרת שימוש
Special Warning עלון לרופא
Physicians Leaflet
Special Warning : אזהרת שימוש
4.4 Special warnings and precautions for use Bradyarrhythmia Initiation of treatment with ozanimod Prior to treatment initiation with ozanimod, an ECG in all patients should be obtained to determine whether any pre-existing cardiac abnormalities are present. In patients with certain pre-existing conditions, first-dose monitoring is recommended (see below). Initiation of ozanimod may result in transient reductions in heart rate (HR) (see sections 4.8 and 5.1), and, therefore the initial dose escalation regimen to reach the maintenance dose (0.92 mg) on day 8 should be followed (see section 4.2). After the initial dose of ozanimod 0.23 mg, the HR decrease started at Hour 4, with the greatest mean reduction at Hour 5, returning towards baseline at Hour 6. With continued dose escalation, there were no clinically relevant HR decreases. Heart rates below 40 beats per minute were not observed. If necessary, the decrease in HR induced by ozanimod can be reversed by parenteral doses of atropine or isoprenaline. Caution should be applied when ozanimod is initiated in patients receiving treatment with a beta- blocker or a calcium-channel blocker (e.g. diltiazem and verapamil) because of the potential for additive effects on lowering HR. Beta-blockers and calcium-channel blockers treatment can be initiated in patients receiving stable doses of ozanimod. The co-administration of ozanimod in patients on a beta-blocker in combination with a calcium channel blocker has not been studied (see section 4.5). First dose monitoring in patients with certain pre-existing cardiac conditions Due to the risk of transient decreases in HR with the initiation of ozanimod, first-dose, 6-hour monitoring for signs and symptoms of symptomatic bradycardia is recommended in patients with resting HR < 55 bpm, second-degree [Mobitz type I] AV block or a history of myocardial infarction or heart failure (see section 4.3). Patients should be monitored with hourly pulse and blood pressure measurement during this 6-hour period. An ECG prior to and at the end of this 6-hour period is recommended. Additional monitoring is recommended in patients if at hour 6 post-dose: • heart rate is less than 45 bpm • heart rate is the lowest value post-dose, suggesting that the maximum decrease in HR may not have occurred yet • there is evidence of a new onset second-degree or higher AV block at the 6-hour post-dose ECG • QTc interval ≥ 500 msec In these cases, appropriate management should be initiated and observation continued until the symptoms/findings have resolved. If medical treatment is required, monitoring should be continued overnight, and a 6-hour monitoring period should be repeated after the second dose of ozanimod. Cardiologist advice should be obtained before initiation of ozanimod in the following patients to decide if ozanimod can safely be initiated and to determine the most appropriate monitoring strategy • history of cardiac arrest, cerebrovascular disease, uncontrolled hypertension, or severe untreated sleep apnoea, history of recurrent syncope or symptomatic bradycardia; • pre-existing significant QT interval prolongation (QTc greater than 500 msec) or other risks for QT prolongation, and patients on medicinal products other than beta-blockers and calcium- channel blockers that may potentiate bradycardia; • Patients on class Ia (e.g. quinidine, disopyramide) or class III (e.g. amiodarone, sotalol) antiarrhythmic medicinal products, which have been associated with cases of torsades de pointes in patients with bradycardia have not been studied with ozanimod. Liver injury Elevations of aminotransferases, gamma-glutamyl transferase (GGT) and bilirubin have been reported in patients treated with ozanimod (see section 4.8). Clinically significant liver injury has occurred in patients treated with ozanimod in the post marketing setting. Signs of liver injury, including elevated serum hepatic enzymes and elevated total bilirubin, have occurred as early as ten days after the first dose. Severe liver injury may result in the need for a liver transplant (see section 4.8). Recent (i.e. within last 6 months) transaminase and bilirubin levels should be available before initiation of treatment with ozanimod. In the absence of clinical symptoms, liver transaminases and bilirubin levels should be monitored at Months 1, 3, 6, 9 and 12 on therapy and periodically thereafter. If liver transaminases rise above 5 times the ULN, more frequent monitoring including serum bilirubin and alkaline phosphatase (ALP) should be instituted. If liver transaminases above 5 times the ULN are confirmed, or at least 3 times the ULN associated with increase of serum bilirubin more than 2 times the ULN, treatment with ozanimod should be interrupted and only re-commenced once liver transaminase values have normalised (including if an alternative cause of the hepatic dysfunction is discovered). Patients who develop symptoms suggestive of hepatic dysfunction, such as unexplained nausea, vomiting, abdominal pain, fatigue, anorexia, or jaundice and/or dark urine, should have hepatic enzymes checked and ozanimod should be discontinued if significant liver injury is confirmed. Resumption of therapy will be dependent on whether another cause of liver injury is determined and on the benefits to patient of resuming therapy versus the risks of recurrence of liver dysfunction. Patients with pre-existing liver disease may be at increased risk of developing elevated hepatic enzymes when taking ozanimod (see section 4.2). Ozanimod has not been studied in patients with severe pre-existing hepatic injury (Child-Pugh class C) and must not be used in these patients (see section 4.3). Immunosuppressive effects Ozanimod has an immunosuppressive effect that predisposes patients to a risk of infection, including opportunistic infections, and may increase the risk of developing malignancies, including those of the skin. Physicians should carefully monitor patients, especially those with concurrent conditions or known factors, such as previous immunosuppressive therapy. If this risk is suspected, discontinuation of treatment should be considered by the physician on a case-by-case basis (see section 4.3). Infections Ozanimod causes a mean reduction in peripheral blood lymphocyte count to approximately 45% of baseline values because of reversible retention of lymphocytes in the lymphoid tissues. Ozanimod may, therefore, increase the susceptibility to infections (see section 4.8). A recent (i.e., within 6 months or after discontinuation of prior MS or UC therapy) complete blood cell count (CBC) should be obtained, including lymphocyte count, before initiation of ozanimod. Assessments of CBC are also recommended periodically during treatment. Absolute lymphocyte counts < 0.2 x 109/L, if confirmed, should lead to interruption of ozanimod therapy until the level reaches > 0.5 x 109/L when re-initiation of ozanimod can be considered. The initiation of ozanimod administration in patients with any active infection should be delayed until the infection is resolved. Patients should be instructed to report promptly symptoms of infection to their physician. Effective diagnostic and therapeutic strategies should be employed in patients with symptoms of infection while on therapy. If a patient develops a serious infection, treatment interruption with ozanimod should be considered. Because the elimination of ozanimod after discontinuation may take up to 3 months, monitoring for infections should be continued throughout this period. Prior and concomitant treatment with antineoplastic, non-corticosteroid immunosuppressive, or immune-modulating therapies In MS and UC clinical studies, patients who received ozanimod were not to receive concomitant antineoplastic, non-corticosteroid immunosuppressive (e.g. azathioprine and 6-mercaptopurine in UC), or immune-modulating therapies used for treatment of MS and UC. Concomitant use of ozanimod with any of these therapies would be expected to increase the risk of immunosuppression and should be avoided. In UC clinical studies, concomitant use of corticosteroids was allowed and did not appear to influence the safety or efficacy of ozanimod, however, long-term data on concomitant use of ozanimod and corticosteroids are still limited. When switching to ozanimod from immunosuppressive medicinal products, the half-life and mode of action must be considered to avoid an additive immune effect whilst at the same time minimizing the risk of disease reactivation. Ozanimod can generally be started immediately after discontinuation of interferon (IFN) or glatiramer. Progressive multifocal leukoencephalopathy (PML) PML is an opportunistic viral infection of the brain caused by the John Cunningham virus (JCV) that typically occurs in patients who are immunocompromised and may lead to death or severe disability. PML has been reported in patients treated with S1P receptor modulators, including ozanimod, and other therapies for MS and UC. JCV infection resulting in PML has been associated with some risk factors (e.g., polytherapy with immunosuppressants, severely immunocompromised patients). Typical symptoms associated with PML are diverse, progress over days to weeks, and include progressive weakness on one side of the body or clumsiness of limbs, disturbance of vision, and changes in thinking, memory, and orientation leading to confusion and personality changes. Physicians should be vigilant for clinical symptoms or MRI findings that may be suggestive of PML. MRI findings may be apparent before clinical signs or symptoms. If PML is suspected, treatment with ozanimod should be suspended until PML has been excluded. If confirmed, treatment with ozanimod should be discontinued. Vaccinations No clinical data are available on the efficacy and safety of vaccinations in patients taking ozanimod. The use of live attenuated vaccines should be avoided during and for 3 months after treatment with ozanimod. If live attenuated vaccine immunizations are required, these should be administered at least 1 month prior to initiation of ozanimod. Varicella Zoster Virus (VZV) vaccination of patients without documented immunity to VZV is recommended prior to initiating treatment with ozanimod. Cutaneous neoplasms Half of the neoplasms reported with ozanimod in the MS controlled Phase 3 studies consisted of non-melanoma skin malignancies, with basal cell carcinoma presenting as the most common skin neoplasm and reported with similar incidence rates in the combined ozanimod (0.2%, 3 patients) and IFN ß-1a (0.1%, 1 patient) groups. In patients treated with ozanimod in UC controlled clinical studies one patient (0.2%) had squamous cell carcinoma of the skin, in the induction period, and one patient (0.4%) had basal cell carcinoma, in the maintenance period. There were no cases in patients who received placebo. Since there is a potential risk of malignant skin growths, patients treated with ozanimod should be cautioned against exposure to sunlight without protection. These patients should not receive concomitant phototherapy with UV-B-radiation or PUVA-photochemotherapy. Macular oedema Macular oedema with or without visual symptoms was observed with ozanimod (see section 4.8) in patients with pre-existing risk factors or comorbid conditions. Patients with a history of uveitis or diabetes mellitus or underlying/co existing retinal disease are at increased risk of macular oedema (see section 4.8). It is recommended that patients with diabetes mellitus, uveitis or a history of retinal disease undergo an ophthalmological evaluation prior to treatment initiation with ozanimod and have follow up evaluations while receiving therapy. Patients who present with visual symptoms of macular oedema should be evaluated and, if confirmed, treatment with ozanimod should be discontinued. A decision on whether ozanimod should be re- initiated after resolution needs to take into account the potential benefits and risks for the individual patient. Posterior reversible encephalopathy syndrome (PRES) PRES is a syndrome characterised by sudden onset of severe headache, confusion, seizures and visual loss. Symptoms of PRES are usually reversible but may evolve into ischaemic stroke or cerebral haemorrhage. In MS controlled clinical trials with ozanimod, one case of PRES was reported in a patient with Guillain-Barré syndrome. If PRES is suspected, treatment with ozanimod should be discontinued. Blood pressure effects In MS and UC controlled clinical studies, hypertension was more frequently reported in patients treated with ozanimod than in patients treated with IFN β-1a IM (MS) or placebo (UC) and in patients receiving concomitant ozanimod and SSRIs or SNRIs (see section 4.8). Blood pressure should be regularly monitored during treatment with ozanimod. Respiratory effects Ozanimod should be used with caution in patients with severe respiratory disease, pulmonary fibrosis and chronic obstructive pulmonary disease. Concomitant medicinal products The coadministration with inhibitors of monoamine oxidase (MAO), or CYP2C8 inducer (rifampicin) with ozanimod is not recommended (see section 4.5). Women of childbearing potential Due to risk to the foetus, ozanimod is contraindicated during pregnancy and in women of childbearing potential not using effective contraception. Before initiation of treatment, women of childbearing potential must be informed of this risk to the foetus, must have a negative pregnancy test and must use effective contraception during treatment, and for 3 months after treatment discontinuation (see sections 4.3 and 4.6 and the information contained in the Prescriber’s checklist). Return of MS disease activity after ozanimod discontinuation Severe exacerbation of disease, including disease rebound, has been rarely reported after discontinuation of another S1P receptor modulator. In the ozanimod long-term extension study, following permanent discontinuation of ozanimod, clinical relapses were reported in 3.3% of patients, none with severe exacerbation of disease or severe increase in disability. Patients should be observed for return of disease activity upon ozanimod discontinuation and appropriate treatment should be instituted as required. Sodium content This medicinal product contains less than 1 mmol sodium (23 mg) per capsule, that is to say essentially ‘sodium-free’.
Effects on Driving
4.7 Effects on ability to drive and use machines Zeposia has no or negligible influence on the ability to drive and use machines.

פרטי מסגרת הכללה בסל
התרופה האמורה תינתן לטיפול במקרים האלה:א. כמונותרפיה לטיפול בחולים עם אבחנה וודאית של טרשת נפוצה (על פי הקריטריונים העדכניים על שם McDonald) עם מחלה פעילה או Clinically isolated syndrome (CIS), בהתאם לתנאי הרישום. הטיפול לא יינתן לחולים עם מחלה פרוגרסיבית ראשונית (PPMS) או פרוגרסיבית שניונית פעילה (SPMS) שאינם מטופלים בתרופות ייעודיות לטרשת נפוצה.התחלת הטיפול בתרופה תיעשה לפי מרשם של נוירו אימונולוג שעבר השתלמות עמיתים, או נוירולוג ילדים שעבר השתלמות עמיתים בטרשת נפוצה, או מומחה בנוירולוגיה העובד במרפאת טרשת נפוצה או מרפאה נוירואימונולוגית ייעודית.ב. מחלת מעי דלקתית מסוג Ulcerative colitis בחולים שמיצו טיפול קודם – טיפול לא ביולוגי או טיפול ביולוגי.
שימוש לפי פנקס קופ''ח כללית 1994
לא צוין
תאריך הכללה מקורי בסל
01/03/2021
הגבלות
תרופה מוגבלת לרישום ע'י רופא מומחה או הגבלה אחרת
רישום
167 34 36600 99
מחיר
0 ₪
מידע נוסף
עלון מידע לצרכן
14.02.22 - עלון לצרכן אנגלית 14.02.22 - עלון לצרכן עברית 14.02.22 - עלון לצרכן ערבית 14.02.22 - עלון לצרכן 12.07.21 - עלון לצרכן אנגלית 12.07.21 - עלון לצרכן עברית 12.07.21 - עלון לצרכן ערבית 02.09.21 - עלון לצרכן 09.03.22 - עלון לצרכן אנגלית 09.03.22 - עלון לצרכן עברית 09.03.22 - עלון לצרכן ערבית 12.07.21 - עלון לצרכן אנגלית 28.12.23 - עלון לצרכן עברית 17.01.24 - עלון לצרכן עברית 19.01.24 - עלון לצרכן אנגלית 19.01.24 - עלון לצרכן עברית 19.01.24 - עלון לצרכן ערבית 31.03.24 - עלון לצרכן אנגלית 31.03.24 - עלון לצרכן עברית 31.03.24 - עלון לצרכן ערבית 31.03.24 - עלון לצרכן 31.03.24 - עלון לצרכן 03.05.24 - עלון לצרכן עברית 21.06.24 - עלון לצרכן עברית 25.06.24 - עלון לצרכן אנגלית 25.06.24 - עלון לצרכן עברית 25.06.24 - עלון לצרכן ערבית 22.07.24 - עלון לצרכן אנגלית 22.07.24 - עלון לצרכן עברית 22.07.24 - עלון לצרכן ערבית 22.07.24 - עלון לצרכן 25.07.24 - עלון לצרכן עברית 10.08.24 - עלון לצרכן אנגלית 10.08.24 - עלון לצרכן עברית 10.08.24 - עלון לצרכן ערבית 04.01.22 - החמרה לעלון 09.03.22 - החמרה לעלון 28.12.23 - החמרה לעלון 03.05.24 - החמרה לעלון 25.07.24 - החמרה לעלוןלתרופה במאגר משרד הבריאות
זפוסיה 0.46 מ"ג