Quest for the right Drug
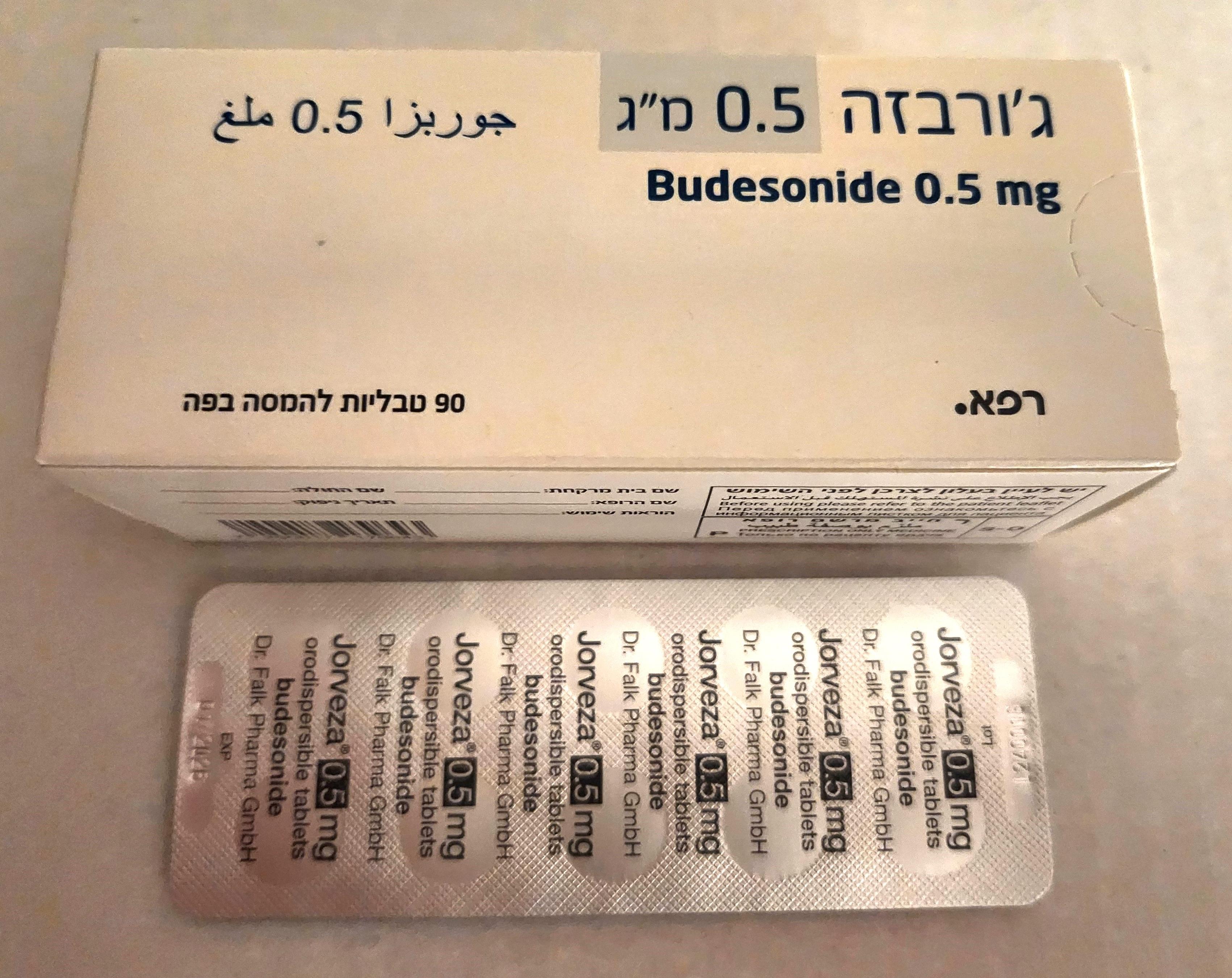
ג'ורבזה 0.5 מ"ג JORVEZA 0.5 MG (BUDESONIDE)
תרופה במרשם
תרופה בסל
נרקוטיקה
ציטוטוקסיקה
צורת מתן:
פומי : PER OS
צורת מינון:
טבליות מסיסות בפה : TABLETS ORODISPERSIBLE
עלון לרופא
מינוניםPosology התוויות
Indications תופעות לוואי
Adverse reactions התוויות נגד
Contraindications אינטראקציות
Interactions מינון יתר
Overdose הריון/הנקה
Pregnancy & Lactation אוכלוסיות מיוחדות
Special populations תכונות פרמקולוגיות
Pharmacological properties מידע רוקחי
Pharmaceutical particulars אזהרת שימוש
Special Warning עלון לרופא
Physicians Leaflet
Posology : מינונים
4.2 Posology and method of administration The treatment with this medicinal product should be initiated by a gastroenterologist or a physician experienced in the diagnosis and treatment of eosinophilic esophagitis. Posology Induction of remission The recommended daily dose is 2 mg budesonide as one 1-mg-tablet in the morning and one 1 mg tablet in the evening. The usual duration of induction treatment is 6 weeks. For patients who are not appropriately responding during 6 weeks the treatment can be extended to up to 12 weeks. Maintenance of remission The recommended daily dose is 1 mg budesonide as one 0.5-mg-tablet in the morning and one 0.5 mg tablet in the evening, or 2 mg budesonide as 1-mg-tablet in the morning and one 1-mg-tablet in the evening, depending on the individual clinical requirement of the patient. A maintenance dose of 1 mg budesonide twice daily is recommended for patients with long standing disease history and/or high extent of esophageal inflammation in their acute disease state, see also section 5.1. The duration of maintenance therapy is determined by the treating physician. Special populations Renal impairment There are currently no data available for patients with renal impairment. Because budesonide is not excreted via the kidneys, patients with mild to moderate impairment may be treated with caution with the same doses as patients without renal impairment. Budesonide is not recommended for use in patients with severe renal impairment. Hepatic impairment During treatment of patients with hepatic impairment with other budesonide containing medicinal products, budesonide levels were increased. However, no systematic study investigating different levels of hepatic impairment is available. Patients with hepatic impairment should not be treated (see sections 4.4 and 5.2). Paediatric population The safety and efficacy of Jorveza in children and adolescents under the age of 18 years have not been established. No data are available. Method of administration Oral use. The orodispersible tablet should be taken immediately once removed from the blister package. The orodispersible tablet should be taken after a meal. It should be placed on the tip of the tongue and gently pressed against the top of the mouth, where it will disintegrate. This will usually take at least two minutes but can take up to 20 minutes. The effervescence process of the tablet starts after Joreveza comes into contact with saliva and stimulates the production of further saliva. The budesonide-loaded saliva should be swallowed little by little while the orodispersible tablet disintegrates. The orodispersible tablet should not be taken with liquid or food. There should be at least 30 minutes before eating or drinking or performing oral hygiene. Any oral solutions, sprays or chewable tablets should be used at least 30 minutes before or after administration of Jorveza. The orodispersible tablet should not be chewed or swallowed undissolved. These measures ensure optimal exposure of the esophageal mucosa to the active substance by using the adhesive characteristics of mucins in the saliva.

פרטי מסגרת הכללה בסל
א. התרופה תינתן לטיפול במבוגרים עם דלקת ושט אאוזינופילית (Eosinophilic esophagitis) לאחר כישלון בטיפול בתכשיר ממשפחת Proton pump inhibitors.ב. תחילת הטיפול בתרופה האמורה ייעשה לפי מרשם של רופא מומחה בגסטרואנטרולוגיה.
שימוש לפי פנקס קופ''ח כללית 1994
לא צוין
תאריך הכללה מקורי בסל
01/03/2021
הגבלות
תרופה מוגבלת לרישום ע'י רופא מומחה או הגבלה אחרת
מידע נוסף
