Quest for the right Drug
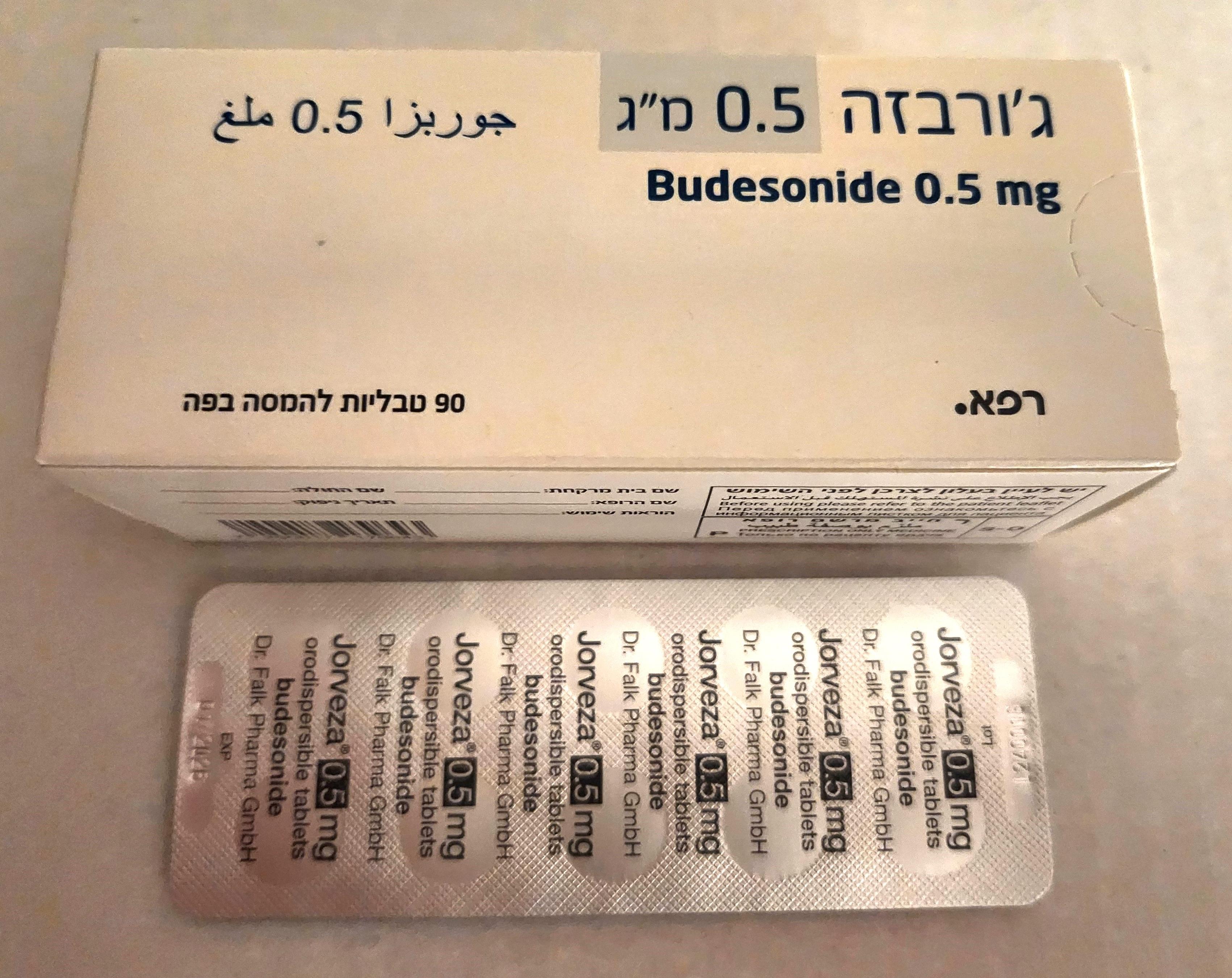
ג'ורבזה 0.5 מ"ג JORVEZA 0.5 MG (BUDESONIDE)
תרופה במרשם
תרופה בסל
נרקוטיקה
ציטוטוקסיקה
צורת מתן:
פומי : PER OS
צורת מינון:
טבליות מסיסות בפה : TABLETS ORODISPERSIBLE
עלון לרופא
מינוניםPosology התוויות
Indications תופעות לוואי
Adverse reactions התוויות נגד
Contraindications אינטראקציות
Interactions מינון יתר
Overdose הריון/הנקה
Pregnancy & Lactation אוכלוסיות מיוחדות
Special populations תכונות פרמקולוגיות
Pharmacological properties מידע רוקחי
Pharmaceutical particulars אזהרת שימוש
Special Warning עלון לרופא
Physicians Leaflet
Special Warning : אזהרת שימוש
4.4 Special warnings and precautions for use Infections Suppression of the inflammatory response and immune function increases the susceptibility to infections and their severity. Symptoms of infections can be atypical or masked. In clinical studies conducted with Jorveza oral, oropharyngeal and esophageal candida infections have been observed with a high frequency (see section 4.8). If indicated, symptomatic candidiasis of the mouth and throat can be treated with topical or systemic anti-fungal therapy whilst still continuing treatment with Jorveza. Chickenpox, herpes zoster and measles can have a more serious course in patients treated with glucocorticosteroids. In patients who have not had these diseases, the vaccination status should be checked, and particular care should be taken to avoid exposure. Vaccines The co-administration of live vaccines and glucocorticosteroids should be avoided as this is likely to reduce the immune response to vaccines. The antibody response to other vaccines may be diminished. Special populations Patients with tuberculosis, hypertension, diabetes mellitus, osteoporosis, peptic ulcer, glaucoma, cataract, family history of diabetes or family history of glaucoma may be at higher risk of experiencing systemic glucocorticosteroid adverse reactions (see below and section 4.8) and should therefore be monitored for the occurrence of such effects. Reduced liver function may affect the elimination of budesonide, causing higher systemic exposure. The risk of adverse reactions (systemic glucocorticosteroid effects) will be increased. However, no systematic data are available. Patients with hepatic impairment should therefore not be treated. Systemic effects of glucocorticosteroids Systemic effects of glucocorticosteroids (e.g., Cushing’s syndrome, adrenal suppression, growth retardation, cataract, glaucoma, decreased bone mineral density and a wide range of psychiatric effects) may occur (see also section 4.8). These adverse reactions depend on the duration of treatment, concomitant and previous glucocorticosteroid treatment and the individual sensitivity. Angioedema Angioedema has been reported with the use of Jorveza, mostly as part of allergic reactions which included rash and itching. If signs of angioedema are observed, the treatment should be stopped. Visual disturbance Visual disturbance may be reported with systemic and topical corticosteroid use. If a patient presents with symptoms such as blurred vision or other visual disturbances, the patient should be considered for referral to an ophthalmologist for evaluation of possible causes which may include cataract, glaucoma or rare diseases such as central serous chorioretinopathy (CSCR) which have been reported after use of systemic and topical corticosteroids. Others Glucocorticosteroids may cause suppression of the hypothalamic–pituitary–adrenal (HPA) axis and reduce the stress response. When patients are subject to surgery or other stresses, supplementary systemic glucocorticosteroid treatment is therefore recommended. Concomitant treatment with ketoconazole or other CYP3A4 inhibitors should be avoided (see section 4.5). Interference with serological testing Because adrenal function may be suppressed by treatment with budesonide, an ACTH stimulation test for diagnosing pituitary insufficiency might show false results (low values). Sodium content Jorveza 0.5 mg 1 mg orodispersible tablets contain 52 mg of sodium per daily dose, equivalent to 2.6% of the WHO recommended maximum daily intake of 2 g sodium for an adult.
Effects on Driving
4.7 Effects on ability to drive and use machines Jorveza has no or negligible influence on the ability to drive and use machines.

פרטי מסגרת הכללה בסל
א. התרופה תינתן לטיפול במבוגרים עם דלקת ושט אאוזינופילית (Eosinophilic esophagitis) לאחר כישלון בטיפול בתכשיר ממשפחת Proton pump inhibitors.ב. תחילת הטיפול בתרופה האמורה ייעשה לפי מרשם של רופא מומחה בגסטרואנטרולוגיה.
שימוש לפי פנקס קופ''ח כללית 1994
לא צוין
תאריך הכללה מקורי בסל
01/03/2021
הגבלות
תרופה מוגבלת לרישום ע'י רופא מומחה או הגבלה אחרת
מידע נוסף
