Quest for the right Drug
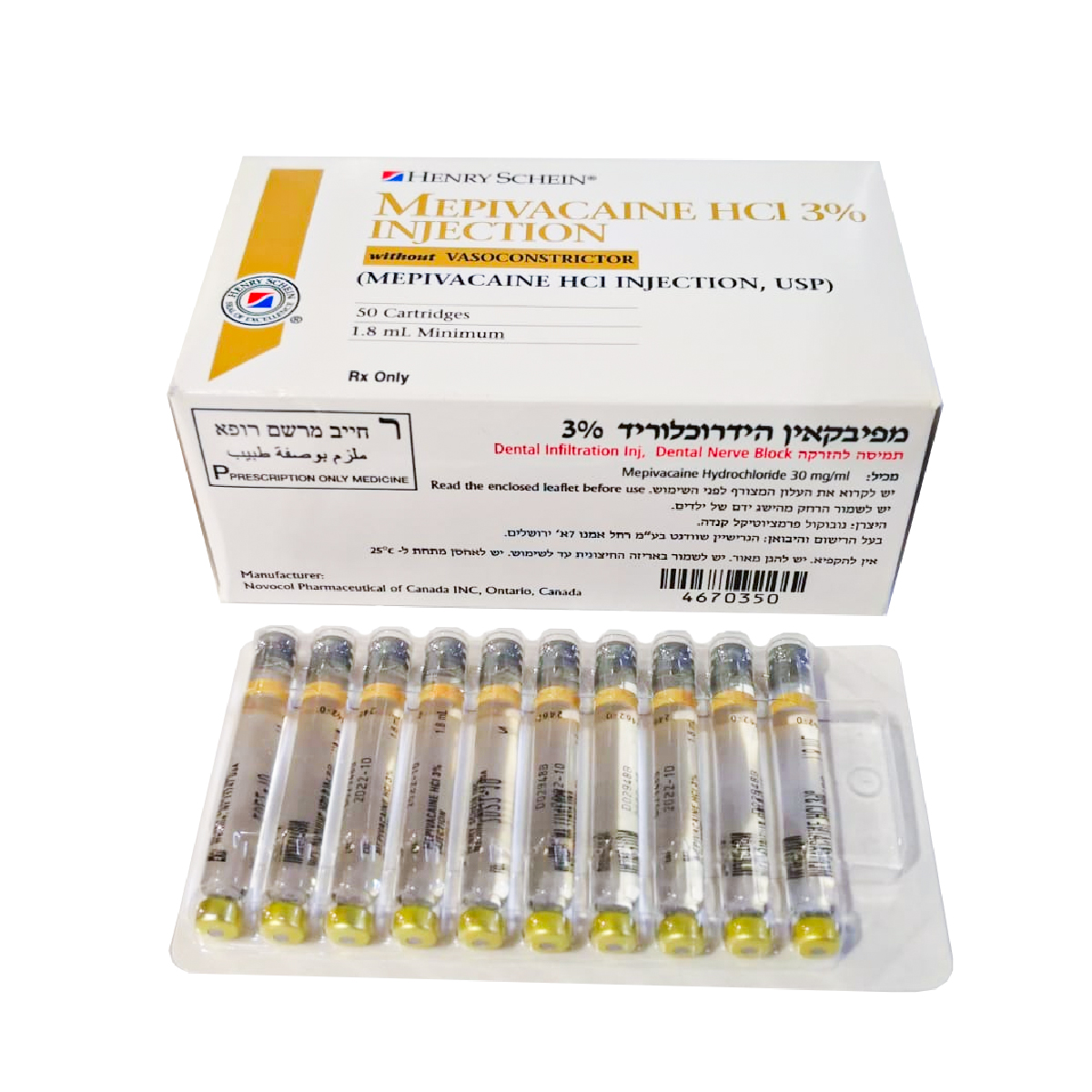
מפיבקאין הידרוכלוריד 3% להזרקה MEPIVACAINE HCL 3% INJECTION (MEPIVACAINE HYDROCHLORIDE)
תרופה במרשם
תרופה בסל
נרקוטיקה
ציטוטוקסיקה
צורת מתן:
הזרקה שינית מוחדרת, חסם עצב דנטלי : DENTAL INFILTRATION INJ., DENTAL NERVE BLOCK
צורת מינון:
תמיסה להזרקה : SOLUTION FOR INJECTION
עלון לרופא
מינוניםPosology התוויות
Indications תופעות לוואי
Adverse reactions התוויות נגד
Contraindications אינטראקציות
Interactions מינון יתר
Overdose הריון/הנקה
Pregnancy & Lactation אוכלוסיות מיוחדות
Special populations תכונות פרמקולוגיות
Pharmacological properties מידע רוקחי
Pharmaceutical particulars אזהרת שימוש
Special Warning עלון לרופא
Physicians Leaflet
Adverse reactions : תופעות לוואי
4.8) Undesirable effects: Summary of the safety profile: Adverse reactions following administration of MEPIVACAINE HCI 3% INJECTION are similar to those observed with other local amide anaesthetics. These adverse reactions are, in general, dose- related and may result from high plasma levels caused by overdose, rapid absorption or unintended intra-vascular injection. They may also result from hypersensitivity, idiosyncrasy, or diminished tolerance by patient. Serious adverse experiences are generally systemic. Tabulated list of adverse reactions: The reported adverse effects come from spontaneous reporting and literature. The frequencies classification follows the convention: Very common (≥1/10), Common (≥1/100 to <1/10), Uncommon (≥1/1,000 to <1/100), Rare (≥1/10,000 to <1/1,000) and Very rare (<1/10,000). Frequency “Not known”: “Not known (cannot be estimated from the available data)”. MedDRA System Organ Frequency Adverse reactions Class Hypersensitivity Immune system Rare Anaphylatic / anaphylactoid disorders reactions Angioedema (Face / tongue / lip / throat / larynx 1 / periorbital oedema) Bronchospasm / asthma 2 Urticaria Euphoric mood Psychiatric disorders Not Known Anxiety/Nervousness 3 Common Headache Neuropathy 4: Neuralgia (Neuropathic pain) Paresthesia (i.e., burning, prickling, itching, tingling, local sensation of heat or cold, with no apparent physical cause) of oral and perioral structures Hypoesthesia / numbness (oral and perioral) Dysesthesia (oral and perioral), including dysgeusia (e.g., taste metallic, taste distorted), ageusia Nervous system Rare Dizziness (light disorders headedness) Tremor 3 Deep CNS depression: Loss of consciousness Coma Convulsion (including tonic- clonic seizure) Presyncope, syncope; Confusional state, disorientation Speech disorder 3 (e.g., dysarthria, logorrhea) Restlessness / agitation 3 Balance disorder (disequilibrium) Somnolence Not known Nystagmus Visual impairment Rare Vision blurred Accommodation disorder Eye disorders Horner’s syndrome Not known Eyelid ptosis Enophthalmos Diplopia (paralysis of oculomotor muscles) Amaurosis (blindness) Mydriasis Miosis Rare Vertigo Ear and labyrinth Ear discomfort disorders Not Known Tinnitus Hyperacusis Cardiac arrest Bradyarrhythmia Bradycardia Tachyarrhythmia (including ventricular extrasystoles Rare and ventricular fibrillation) 5 Cardiac disorders Angina pectoris 6 Conduction disorders (atrioventricular block) Tachycardia Palpitations Not known Myocardial depression Hypotension (with possible Rare circulatory collapse) Very rare Hypertension Vascular disorders Vasodilatation Not known Local / Regional hyperaemia Respiratory depression Bradypnoea Apnoea (respiratory arrest) Rare Yawning Respiratory, thoracic Dyspnoea 2 and mediastinal Tachypnea disorders Hypoxia 7 (including cerebral) Not known Hypercapnia 7 Dysphonia (Hoarseness 1) Nausea Vomiting Gingival / oral mucosal Rare exfoliation (sloughing) / Gastrointestinal ulceration disorders Swelling 8 of tongue, lip, gums Stomatitis, glossitis, Not known gingivitis Salivary hypersecretion Rash (eruption) Skin and subcutaneous Rare Erythema tissue disorders Pruritus Swelling face Hyperhidrosis (sweating or perspiration) Musculoskeletal and Muscle twitching connective tissue Rare Chills (shivering) disorders General disorders and Local swelling administration site Rare Injection site swelling conditions Chest pain Fatigue, asthenia Not known (weakness) Feeling hot Injection site pain Injury, poisoning and procedural Not known Nerve injury complications Description of selected adverse reactions: 1 laryngo-pharyngeal oedema may characteristically occur with hoarseness and/or dysphagia; 2 bronchospasm (bronchoconstriction) may characteristically occur with dyspnoea; 3 several adverse events, like agitation, anxiety / nervousness tremor, speech disorder may be warning signs before CNS depression. In attendance of these signs, patients should be requested to hyperventilate and surveillance should be instituted (see Section 4.9.) 4 neural pathologies that may occur with the various symptoms of abnormal sensations (i.e., paresthesia, hypoesthesia, dysesthesia, hyperesthesia, etc) of the lips, tongue and oral tissues. These data originated in post-marketing reports, mostly following nerve blocks in mandible, involving various branches of the trigeminal nerve; 5 mostly in patients with underlying cardiac disease or those receiving certain drugs; 6 in predisposed patients or those with risk factors of ischemic heart disease; 7 hypoxia and hypercapnia are secondary to respiratory depression and / or to seizures and sustained muscular exertion; 8 by accidental biting or chewing of the lips or tongue while the anaesthesia persists. Reporting of suspected adverse reactions: Reporting suspected adverse reactions after authorisation of the medicinal product is important. It allows continued monitoring of the benefit / risk balance of the medicinal product. Any suspected adverse events should be reported to the Ministry of Health according to the National Regulation by using an online form: https://sideeffects.health.gov.il.

שימוש לפי פנקס קופ''ח כללית 1994
לא צוין
תאריך הכללה מקורי בסל
01/01/1995
הגבלות
תרופה שאושרה לשימוש כללי בקופ'ח
מידע נוסף
