Quest for the right Drug
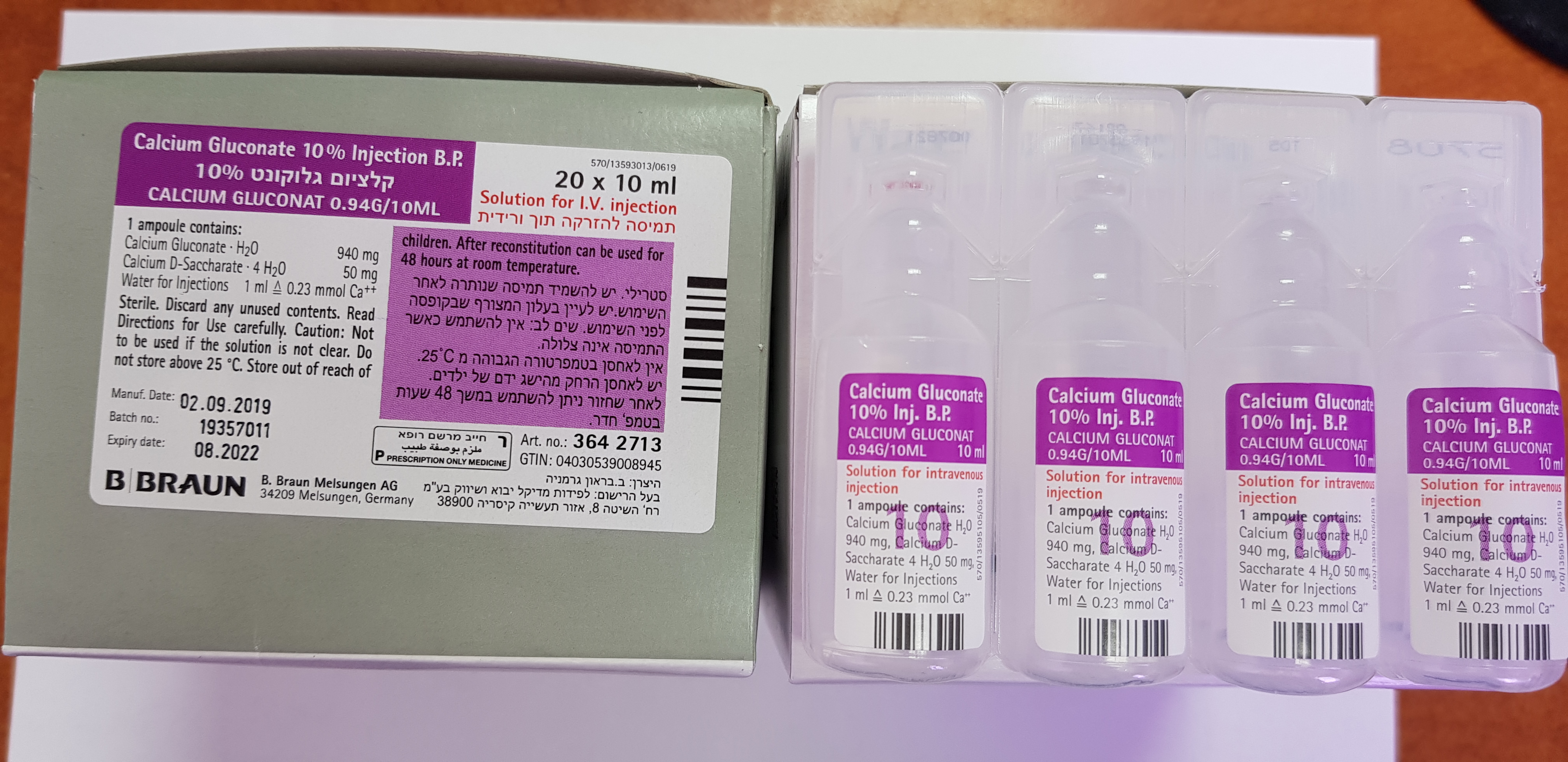
קלציום גלוקונט % 10 CALCIUM GLUCONATE 10 % INJECTION B.P. (CALCIUM GLUCONATE)
תרופה במרשם
תרופה בסל
נרקוטיקה
ציטוטוקסיקה
צורת מתן:
תוך-ורידי : I.V
צורת מינון:
תמיסה להזרקה : SOLUTION FOR INJECTION
עלון לרופא
מינוניםPosology התוויות
Indications תופעות לוואי
Adverse reactions התוויות נגד
Contraindications אינטראקציות
Interactions מינון יתר
Overdose הריון/הנקה
Pregnancy & Lactation אוכלוסיות מיוחדות
Special populations תכונות פרמקולוגיות
Pharmacological properties מידע רוקחי
Pharmaceutical particulars אזהרת שימוש
Special Warning עלון לרופא
Physicians Leaflet
Adverse reactions : תופעות לוואי
4.8 Undesirable effects The frequency of undesirable effects listed below is defined using the following convention: Very common ≥1/10 Common ≥1/100 to <1/10 Uncommon ≥1/1,000 to <1/100 Rare ≥1/10,000 to <1/1,000 Very rare <1/10,000 Not known Frequency cannot be estimated from the available data. Cardiovascular and other systemic undesirable effects are likely to occur as symptoms of acute hypercalcaemia resulting from intravenous overdose or too rapid intravenous injection. Their occurrence and frequency is directly related to the administration rate and the administered dose. Cardiac disorders Not known: Bradycardia, cardiac arrhythmia Vascular disorders Not known: Hypotension, vasodilatation, circulatory collapse (possibly fatal), flushing, mainly after too rapid injection Gastrointestinal disorders Not known: Nausea, vomiting General disorders and administration site conditions Not known: Heat sensations, sweating Not known: Intramuscular injection may be accompanied by pain sensations or erythema. Ceftriaxone-calcium salt precipitation Rarely, severe, and in some cases, fatal, adverse reactions have been reported in pre-term and full-term neonates (aged < 28 days) who had been treated with intravenous ceftriaxone and calcium. Precipitations of ceftriaxone-calcium salt have been observed in lung and kidneys post-mortem. The high risk of precipitation in neonates is a result of their low blood volume and the longer half-life of ceftriaxone compared with adults (see sections 4.3, 4.4, and 6.2). Adverse reactions only occurring with improper administration technique: If intramuscular injection is not performed sufficiently deep intramuscularly, infiltration into the adipose tissue may occur with subsequent abscess formation, tissue induration, and necrosis. Calcinosis cutis, possibly followed by skin ablation and necrosis, due to extravasation, has been reported. Reddening of skin, burning sensation or pain during intravenous injection may indicate accidental perivascular injection, which may lead to tissue necrosis. Reporting of suspected adverse reactions Reporting of suspected adverse reactions after authorisation of the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the medicinal product. Any suspected adverse events should be reported to the Ministry of Health according to the National Regulation by using an online form (https://sideeffects.health.gov.il)

שימוש לפי פנקס קופ''ח כללית 1994
Calcium supplement
תאריך הכללה מקורי בסל
01/01/1995
הגבלות
תרופה שאושרה לשימוש כללי בקופ'ח
מידע נוסף
