Quest for the right Drug
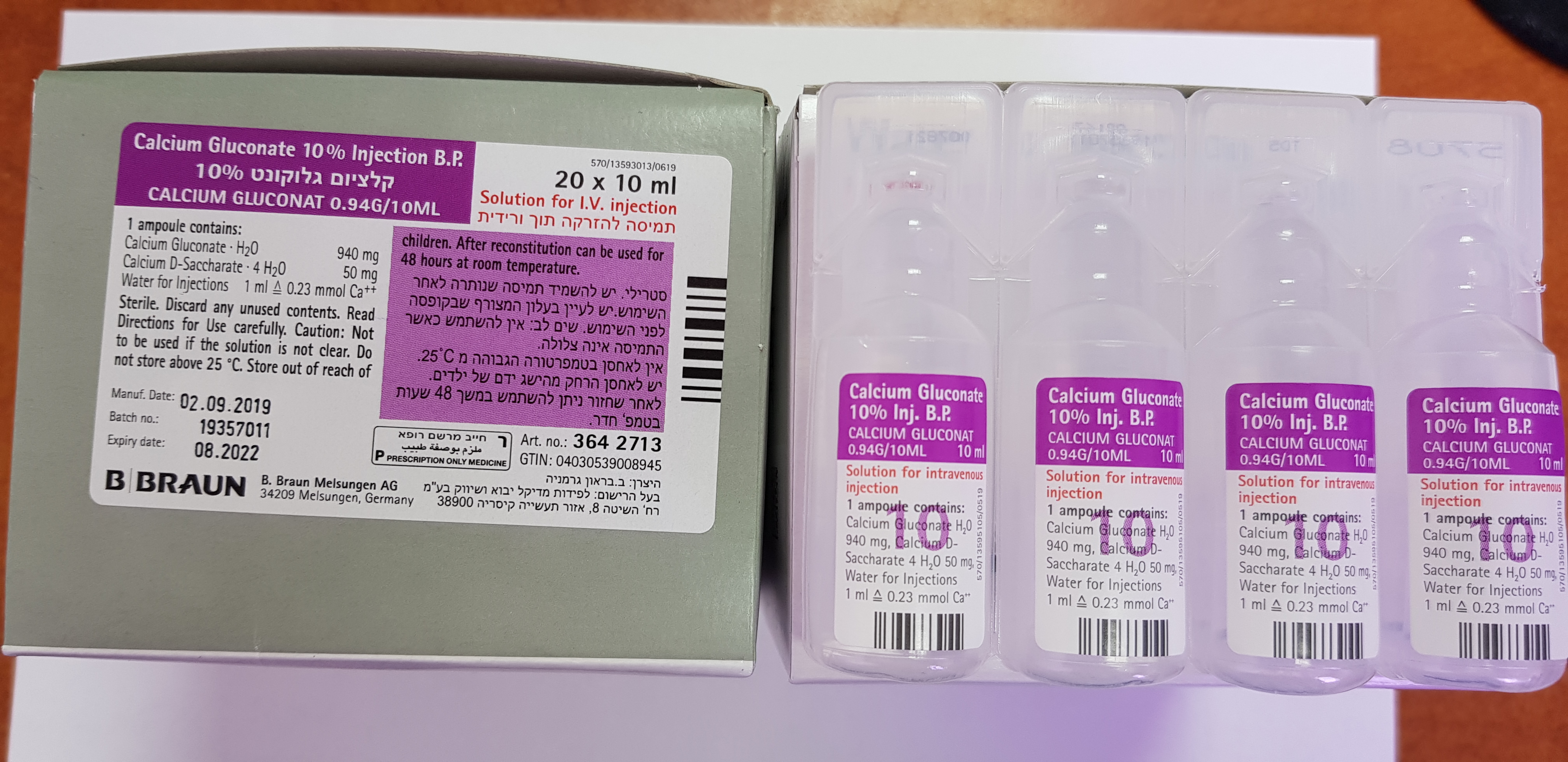
קלציום גלוקונט % 10 CALCIUM GLUCONATE 10 % INJECTION B.P. (CALCIUM GLUCONATE)
תרופה במרשם
תרופה בסל
נרקוטיקה
ציטוטוקסיקה
צורת מתן:
תוך-ורידי : I.V
צורת מינון:
תמיסה להזרקה : SOLUTION FOR INJECTION
עלון לרופא
מינוניםPosology התוויות
Indications תופעות לוואי
Adverse reactions התוויות נגד
Contraindications אינטראקציות
Interactions מינון יתר
Overdose הריון/הנקה
Pregnancy & Lactation אוכלוסיות מיוחדות
Special populations תכונות פרמקולוגיות
Pharmacological properties מידע רוקחי
Pharmaceutical particulars אזהרת שימוש
Special Warning עלון לרופא
Physicians Leaflet
Special Warning : אזהרת שימוש
4.4 Special warnings and precautions for use Special warnings In the exceptional case of intravenous administration of calcium gluconate to patients receiving cardiac glycosides, adequate cardiac monitoring is mandatory and emergency treatment of cardiac complications such as serious arrhythmias must be available. Calcium Gluconate 10% solution for injection/infusion should be administered slowly (in 100 ml glucose 5% over 20 minutes). Rapid calcium administration may precipitate myocardial digoxin toxicity therefore other methods e.g. haemodialysis should also be considered after consultation with specialists. Calcium salts should only be used with caution and after careful establishment of the indication in patients with nephrocalcinosis, heart diseases, sarcoidosis (Boeck's disease), in patients receiving epinephrine (see section 4.5), or in the elderly. Renal impairment may be associated with hypercalcaemia and secondary hyperparathyroidism. Therefore, in patients with renal impairment, parenteral calcium should be administered only after careful assessment of the indication and the calcium-phosphate balance should be monitored. Calcium gluconate and calcium chloride are presented in 10 ml ampoules at 10% (w/v) for injection but are not equivalent in calcium content: - 10 ml of Calcium Gluconate 10 % w/v Injection BP contains 2.23 mmol calcium - 10 ml of calcium chloride 10% solution contains 6.8 mmol of calcium The difference in calcium content should be accounted for to achieve the correct calcium dose when using either salt to avoid medication errors. Patients receiving ceftriaxone In patients of any age ceftriaxone must not be mixed or administered simultaneously with any calcium-containing intravenous solutions even via different infusion lines or different infusion sites (see section 6.2). Cases of fatal reactions with calcium-ceftriaxone precipitates in lungs and kidneys in premature and full-term newborns aged less than 1 month have been described. However, in patients older than 28 days of age ceftriaxone and calcium- containing solutions may be administered sequentially one after another if infusion lines at different sites are used or if the infusion lines are replaced or thoroughly flushed between infusions with physiological salt-solution to avoid precipitation. Sequential infusions of ceftriaxone and calcium-containing products must be avoided in case of hypovolaemia. Calcium Gluconate 10% solution for injection/infusion must not be mixed with or administered through the same intravenous line as sodium bicarbonate due to the risk of precipitation. Precautions for use Solutions containing calcium should be administered slowly to minimise peripheral vasodilation and cardiac depression. Intravenous injections should be accompanied by heart rate or ECG control because bradycardia with vasodilatation or arrhythmia can occur when calcium is administered too quickly. In paediatric patients, Calcium Gluconate 10 % Injection B.P. should not be injected intramuscularly but only slowly intravenously. Patients receiving calcium salts should be monitored carefully to ensure maintenance of correct calcium balance without tissue deposition. Plasma levels and urinary excretion of calcium should be monitored when high- dose parenteral calcium is administered. Calcium Gluconate 10 % Injection B.P. should not be injected in adipose tissue as calcium is insoluble in adipose tissue and may cause infiltration and subsequent abscess formation, tissue indurations and necrosis. After perivascular or superficial intramuscular injection local irritation, possibly followed by skin ablation or tissue necrosis, may occur (see section 4.8). Extravasation must be avoided; the injection site should be monitored carefully. High Vitamin D intake should be avoided.
Effects on Driving
4.7 Effects on ability to drive and use machines Not relevant.

שימוש לפי פנקס קופ''ח כללית 1994
Calcium supplement
תאריך הכללה מקורי בסל
01/01/1995
הגבלות
תרופה שאושרה לשימוש כללי בקופ'ח
מידע נוסף
