Quest for the right Drug
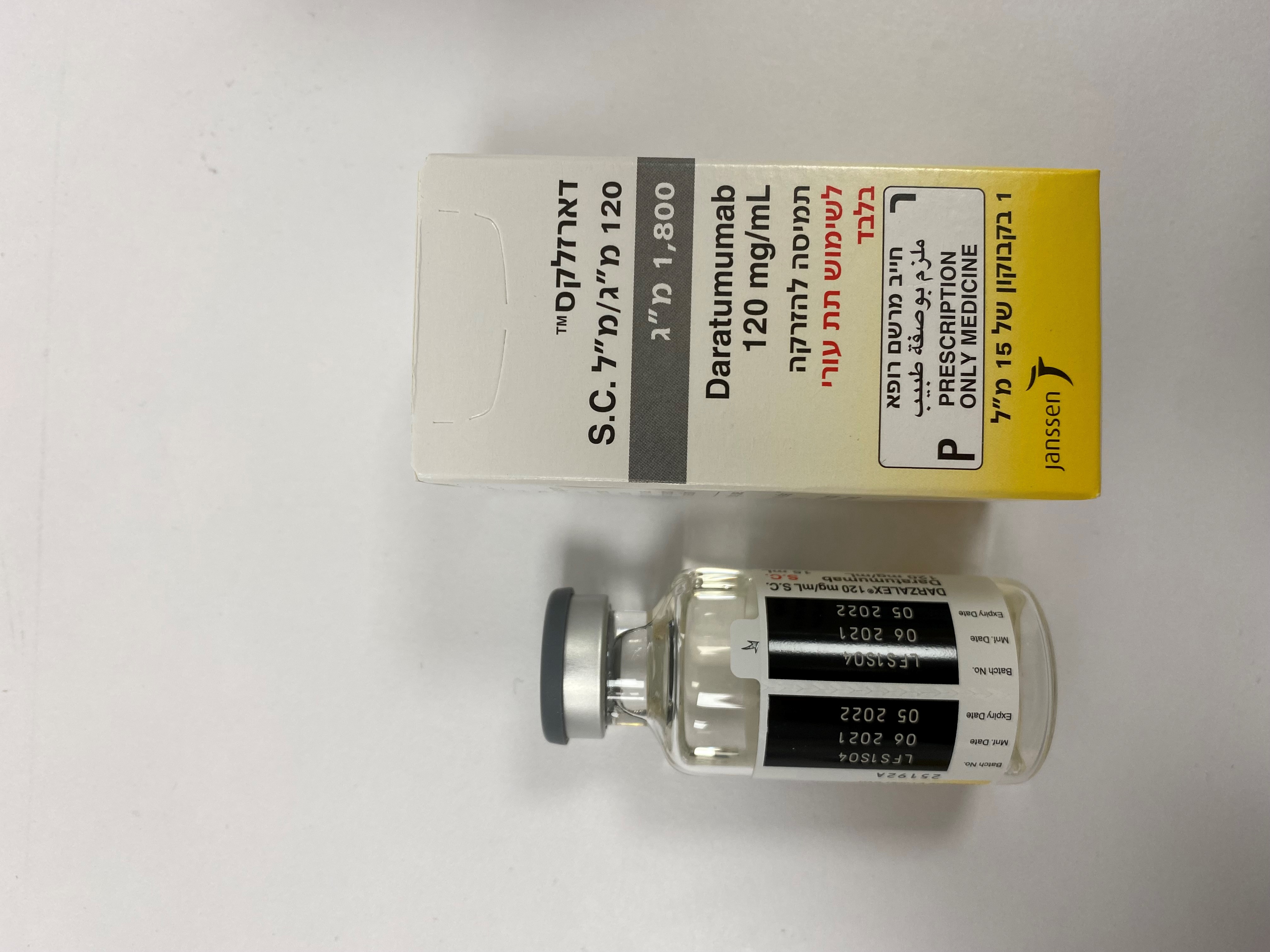
דארזלקס 120 מ"ג/מ"ל תת עורי 1,800 מ"ג DARZALEX 120 MG/ML S.C. 1,800 MG (DARATUMUMAB)
תרופה במרשם
תרופה בסל
נרקוטיקה
ציטוטוקסיקה
צורת מתן:
תת-עורי : S.C
צורת מינון:
תמיסה להזרקה : SOLUTION FOR INJECTION
עלון לרופא
מינוניםPosology התוויות
Indications תופעות לוואי
Adverse reactions התוויות נגד
Contraindications אינטראקציות
Interactions מינון יתר
Overdose הריון/הנקה
Pregnancy & Lactation אוכלוסיות מיוחדות
Special populations תכונות פרמקולוגיות
Pharmacological properties מידע רוקחי
Pharmaceutical particulars אזהרת שימוש
Special Warning עלון לרופא
Physicians Leaflet
Pharmacological properties : תכונות פרמקולוגיות
Pharmacodynamic Properties
5.1 Pharmacodynamic properties Pharmacotherapeutic group: Antineoplastic agents, monoclonal antibodies and antibody drug conjugates, CD38 (Clusters of Differentiation 38) inhibitors, ATC code: L01FC01. DARZALEX 120MG/ML S.C. 1,800MG solution for subcutaneous injection contains recombinant human hyaluronidase (rHuPH20). rHuPH20 works locally and transiently to degrade hyaluronan ((HA), a naturally occurring glycoaminoglycan found throughout the body) in the extracellular matrix of the subcutaneous space by cleaving the linkage between the two sugars (N-acetylglucosamine and glucuronic acid) which comprise HA. rHuPH20 has a half-life in skin of less than 30 minutes. Hyaluronan levels in subcutaneous tissue return to normal within 24 to 48 hours because of the rapid biosynthesis of hyaluronan. Mechanism of action Daratumumab is an IgG1κ human monoclonal antibody (mAb) that binds to the CD38 protein expressed at a high level on the surface of multiple myeloma tumour cells, as well as other cell types and tissues at various levels. CD38 protein has multiple functions such as receptor mediated adhesion, signalling and enzymatic activity. Daratumumab has been shown to potently inhibit the in vivo growth of CD38-expressing tumour cells. Based on in vitro studies, daratumumab may utilise multiple effector functions, resulting in immune mediated tumour cell death. These studies suggest that daratumumab can induce tumour cell lysis through complement-dependent cytotoxicity, antibody-dependent cell-mediated cytotoxicity, and antibody-dependent cellular phagocytosis in malignancies expressing CD38. A subset of myeloid derived suppressor cells (CD38+MDSCs), regulatory T cells (CD38+Tregs) and B cells (CD38+Bregs) are decreased by daratumumab mediated cell lysis. T cells (CD3+, CD4+, and CD8+) are also known to express CD38 depending on the stage of development and the level of activation. Significant increases in CD4+ and CD8+ T cell absolute counts, and percentages of lymphocytes, were observed with daratumumab treatment in peripheral whole blood and bone marrow. In addition, T-cell receptor DNA sequencing verified that T-cell clonality was increased with daratumumab treatment, indicating immune modulatory effects that may contribute to clinical response. Daratumumab induced apoptosis in vitro after Fc mediated cross-linking. In addition, daratumumab modulated CD38 enzymatic activity, inhibiting the cyclase enzyme activity and stimulating the hydrolase activity. The significance of these in vitro effects in a clinical setting, and the implications on tumour growth, are not well-understood. Pharmacodynamic effects Natural killer (NK) cell and T-cell count NK cells are known to express high levels of CD38 and are susceptible to daratumumab mediated cell lysis. Decreases in absolute counts and percentages of total NK cells (CD16+CD56+) and activated (CD16+CD56dim) NK cells in peripheral whole blood and bone marrow were observed with daratumumab treatment. However, baseline levels of NK cells did not show an association with clinical response. Immunogenicity In patients treated with subcutaneous daratumumab in clinical studies, less than 1% of patients developed treatment-emergent anti-daratumumab antibodies. The incidence of treatment-emergent non-neutralizing anti-rHuPH20 antibodies was 7.8% (35/447); with 7.5% (19/255) in the monotherapy DARZALEX 120MG/ML S.C. 1,800MG subcutaneous formulation groups, and 8.3% (16/192) in the pooled combination DARZALEX 120MG/ML S.C. 1,800MG subcutaneous formulation groups. The anti-rHuPH20 antibodies did not appear to impact daratumumab exposures. The clinical relevance of the development of anti-daratumumab or anti-rHuPH20 antibodies after treatment with DARZALEX 120MG/ML S.C. 1,800MG subcutaneous formulation is not known. Clinical experience of DARZALEX 120MG/ML S.C. 1,800MG solution for subcutaneous injection (subcutaneous formulation) Monotherapy – relapsed/refractory multiple myeloma MMY3012, an open-label, randomised, phase III non-inferiority study, compared efficacy and safety of treatment with DARZALEX 120MG/ML S.C. 1,800MG solution for subcutaneous injection (1,800 mg) vs. intravenous (16 mg/kg) daratumumab in patients with relapsed or refractory multiple myeloma who had received at least 3 prior lines of therapy including a proteasome inhibitor (PI) and an immunomodulatory agent (IMiD) or who were double-refractory to a PI and an iMiD. Treatment continued until unacceptable toxicity or disease progression. A total of 522 patients were randomised: 263 to the DARZALEX 120MG/ML S.C. 1,800MG subcutaneous formulation arm and 259 to the intravenous daratumumab arm. The baseline demographic and disease characteristics were similar between the two treatment groups. The median patient age was 67 years (range: 33-92 years), 55% were male and 78% were Caucasian. The median patient weight was 73 kg (range: 29 – 138 kg) Patients had received a median of 4 prior lines of therapy. A total of 51% of patients had prior autologous stem cell transplant (ASCT), 100% of patients were previously treated with both PI(s) and IMiD(s) and most patients were refractory to a prior systemic therapy, including both PI and IMiD (49%). The study met its co-primary endpoints of overall response rate (ORR) by the IMWG response criteria (Table 6) and maximum Ctrough at pre-dose cycle 3 day 1, (see section 5.2). Table 6: Key results from study MMY3012 Subcutaneous Intravenous daratumumab daratumumab (N=263) (N=259) Primary endpoint Overall response (sCR+CR+VGPR+PR), n (%)a 108 (41.1%) 96 (37.1%) 95% CI (%) (35.1%, 47.3%) (31.2%, 43.3%) Ratio of response rates (95% CI)b 1.11 (0.89, 1.37) CR or better, n (%) 5 (1.9%) 7 (2.7%) Very good partial response (VGPR) 45 (17.1%) 37 (14.3%) Partial response (PR) 58 (22.1%) 52 (20.1%) Secondary endpoint Rate of infusion-related reaction, n (%)c 33 (12.7%) 89 (34.5%) Progression-free survival, months Median (95% CI) 5.59 (4.67, 7.56) 6.08 (4.67, 8.31) Hazard ratio (95% CI) 0.99 (0.78, 1.26) a Based on intent-to-treat population. b p-value <0.0001 from Farrington-Manning test for non-inferiority hypothesis. c Based on safety population. P-value<0.0001 from Cochran-Mantel-Haenszel Chi-Squared test. After a median follow-up of 29.3 months, the median OS was 28.2 months (95% CI: 22.8, NE) in the DARZALEX subcutaneous formulation arm and was 25.6 months (95% CI: 22.1, NE) in the intravenous daratumumab arm. Safety and tolerability results, including in lower weight patients, were consistent with the known safety profile for DARZALEX 120MG/ML S.C. 1,800MG subcutaneous formulation and intravenous daratumumab. Results from the modified-CTSQ, a patient reported outcome questionnaire that assesses patient satisfaction with their therapy, demonstrated that patients receiving DARZALEX 120MG/ML S.C. 1,800MG subcutaneous formulation had greater satisfaction with their therapy compared with patients receiving intravenous daratumumab. However, open-label studies are subject to bias. Combination therapies in multiple myeloma MMY2040 was an open-label study evaluating the efficacy and safety of DARZALEX 120MG/ML S.C. 1,800MG subcutaneous formulation 1,800 mg: - in combination with bortezomib, melphalan, and prednisone (D-VMP) in patients with newly diagnosed multiple myeloma (MM) who are ineligible for transplant. Bortezomib was administered by subcutaneous injection at a dose of 1.3 mg/m2 body surface area twice weekly at weeks 1, 2, 4 and 5 for the first 6-week cycle (cycle 1; 8 doses), followed by once weekly administrations at weeks 1, 2, 4 and 5 for eight more 6-week cycles (cycles 2-9; 4 doses per cycle). Melphalan at 9 mg/m2, and prednisone at 60 mg/m2 were orally administered on days 1 to 4 of the nine 6-week cycles (cycles 1-9). DARZALEX 120MG/ML S.C. 1,800MG subcutaneous formulation was continued until disease progression or unacceptable toxicity. - in combination with lenalidomide and dexamethasone (D-Rd) in patients with relapsed or refractory MM. Lenalidomide (25 mg once daily orally on days 1-21 of repeated 28-day [4-week] cycles) was given with low dose dexamethasone 40 mg/week (or a reduced dose of 20 mg/week for patients >75 years or BMI<18.5). DARZALEX 120MG/ML S.C. 1,800MG subcutaneous formulation was continued until disease progression or unacceptable toxicity. - in combination with bortezomib, lenalidomide, and dexamethasone (D-VRd) in patients with newly diagnosed MM who are transplant eligible. Bortezomib was administered by subcutaneous injection at a dose of 1.3 mg/m2 body surface area twice weekly at weeks 1 and 2. Lenalidomide was administered orally at 25 mg once daily on days 1-14; low dose dexamethasone was administered 40 mg/week in 3-week cycles. Total treatment duration was 4 cycles. A total of 199 patients (D-VMP: 67; D-Rd: 65; D-VRd: 67) were enrolled. Efficacy results were determined by computer algorithm using IMWG criteria. The study met its primary endpoint ORR for D-VMP and D-Rd and the primary endpoint VGPR or better for D-VRd (see table 7). Table 7: Efficacy results from study MMY2040 D-VMP (n=67) D-Rd (n=65) D-VRd (n=67) Overall response 60 (89.6%) 61 (93.8%) 65 (97.0%) (sCR+CR+VGPR+PR), n (%)a 90% CI(%) (81.3%, 95.0%) (86.5%, 97.9%) (90.9%, 99.5%) Stringent complete response (sCR) 13 (19.4%) 12 (18.5%) 6 (9.0%) Complete response (CR) 19 (28.4%) 13 (20.0%) 5 (7.5%) Very good partial response (VGPR) 20 (29.9%) 26 (40.0%) 37 (55.2%) Partial response (PR) 8 (11.9%) 10 (15.4%) 17 (25.4%) VGPR or better (sCR + CR + VGPR) 52 (77.6%) 51 (78.5%) 48 (71.6%) 90% CI(%) (67.6%, 85.7%) (68.4%, 86.5%) (61.2%, 80.6%) D-VMP = Daratumumab-bortezomib-melphalan-prednisone; D-Rd = Daratumumab-lenalidomide-dexamethasone; D-VRd = Daratumumab-bortezomib-lenalidomide-dexamethasone; Daratumumab = DARAZALEX subcutaneous formulation; CI=confidence interval. a Based on treated subjects Clinical experience with daratumumab concentrate for solution for infusion (intravenous formulation) Newly diagnosed multiple myeloma Combination treatment with lenalidomide and dexamethasone in patients ineligible for autologous stem cell transplant: Study MMY3008, an open-label, randomised, active-controlled phase III study, compared treatment with intravenous daratumumab 16 mg/kg in combination with lenalidomide and low-dose dexamethasone (DRd) to treatment with lenalidomide and low-dose dexamethasone (Rd) in patients with newly diagnosed multiple myeloma. Lenalidomide (25 mg once daily orally on days 1-21 of repeated 28-day [4-week] cycles) was given with low dose oral or intravenous dexamethasone 40 mg/week (or a reduced dose of 20 mg/week for patients >75 years or body mass index [BMI] <18.5). On intravenous daratumumab infusion days, the dexamethasone dose was given as a pre-infusion medicinal product. Dose adjustments for lenalidomide and dexamethasone were applied according to manufacturer’s prescribing information. Treatment was continued in both arms until disease progression or unacceptable toxicity. A total of 737 patients were randomised: 368 to the DRd arm and 369 to the Rd arm. The baseline demographic and disease characteristics were similar between the two treatment groups. The median age was 73 (range: 45-90) years, with 44% of the patients ≥75 years of age. The majority were white (92%), male (52%), 34% had an Eastern Cooperative Oncology Group (ECOG) performance score of 0, 49.5% had an ECOG performance score of 1 and 17% had an ECOG performance score of ≥2. Twenty-seven percent had International Staging System (ISS) stage I, 43% had ISS stage II and 29% had ISS stage III disease. Efficacy was evaluated by progression free survival (PFS) based on International Myeloma Working Group (IMWG) criteria and overall survival (OS) . With a median follow-up of 28 months, the primary analysis of PFS in study MMY3008 showed an improvement in the DRd arm as compared to the Rd arm; the median PFS had not been reached in the DRd arm and was 31.9 months in the Rd arm (hazard ratio [HR]=0.56; 95% CI: 0.43, 0.73; p < 0.0001), representing 44% reduction in the risk of disease progression or death in patients treated with DRd. Results of an updated PFS analysis after a median follow-up of 64 months continued to show an improvement in PFS for patients in the DRd arm compared with the Rd arm. Median PFS was 61.9 months in the DRd arm and 34.4 months in the Rd arm (HR=0.55; 95% CI: 0.45, 0.67). Figure 1: Kaplan-Meier curve of PFS in study MMY3008 Figure 1: Kaplan-Meier curve of PFS in study MMY3008 With a median follow-up of 56 months, DRd has shown an OS advantage over the Rd arm (HR=0.68; 95% CI: 0.53, 0.86; p=0.0013). Results of an updated OS analysis after a median of 64 months continued to show an improvement in OS for patients in the DRd arm compared to the Rd arm. Median OS was not reached in the DRd arm and was 65.5 months in the Rd arm (HR= 0.66; 95% CI: 0.53, 0.83). Figure 2 Kaplan-Meier curve of OS in study MMY3008 Additional efficacy results from study MMY3008 are presented in table 8 below. Table 8: Additional efficacy results from study MMY3008a DRd (n=368) Rd (n=369) Overall response (sCR+CR+VGPR+PR) n(%) a 342 (92.9%) 300 (81.3%) p-valueb <0.0001 Stringent complete response (sCR) 112 (30.4%) 46 (12.5%) Complete response (CR) 63 (17.1%) 46 (12.5%) Very good partial response (VGPR) 117 (31.8%) 104 (28.2%) Partial response (PR) 50 (13.6%) 104 (28.2%) CR or better (sCR + CR) 175 (47.6%) 92 (24.9%) p-value b <0.0001 VGPR or better (sCR + CR + VGPR) 292 (79.3%) 196 (53.1%) p-valueb <0.0001 MRD negativity ratea,c n(%) 89 (24.2%) 27 (7.3%) 95% CI (%) (19.9%, 28.9%) (4.9%, 10.5%) Odds ratio with 95% CId 4.04 (2.55, 6.39) p-valuee <0.0001 DRd=daratumumab-lenalidomide-dexamethasone; Rd=lenalidomide-dexamethasone; MRD=minimal residual disease; CI=confidence interval a Based on intent-to-treat population b p-value from Cochran Mantel-Haenszel Chi-Squared test. c Based on threshold of 10-5 d Mantel-Haenszel estimate of the odds ratio for un-stratified tables is used. An odds ratio >1 indicates an advantage for DRd. e p-value from Fisherʼs exact test. In responders, the median time to response was 1.05 months (range: 0.2 to 12.1 months) in the DRd group and 1.05 months (range: 0.3 to 15.3 months) in the Rd group. The median duration of response had not been reached in the DRd group and was 34.7 months (95% CI: 30.8, not estimable) in the Rd group. Combination treatment with bortezomib, melphalan and prednisone (VMP) in patients ineligible for autologous stem cell transplant: Study MMY3007, an open-label, randomised, active-controlled phase III study, compared treatment with intravenous daratumumab 16 mg/kg in combination with bortezomib, melphalan and prednisone (D-VMP), to treatment with VMP in patients with newly diagnosed multiple myeloma. Bortezomib was administered by subcutaneous injection at a dose of 1.3 mg/m2 body surface area twice weekly at weeks 1, 2, 4 and 5 for the first 6-week cycle (cycle 1; 8 doses), followed by once weekly administrations at weeks 1, 2, 4 and 5 for eight more 6-week cycles (cycles 2-9; 4 doses per cycle). Melphalan at 9 mg/m2, and prednisone at 60 mg/m2 were orally administered on days 1 to 4 of the nine 6-week cycles (cycles 1-9). Intravenous daratumumab treatment was continued until disease progression or unacceptable toxicity. A total of 706 patients were randomised: 350 to the D-VMP arm and 356 to the VMP arm. The baseline demographic and disease characteristics were similar between the two treatment groups. The median age was 71 (range: 40-93) years, with 30% of the patients ≥75 years of age. The majority were white (85%), female (54%), 25% had an ECOG performance score of 0, 50% had an ECOG performance score of 1 and 25% had an ECOG performance score of 2. Patients had IgG/IgA/Light chain myeloma in 64%/22%/10% of instances, 19% had ISS stage I, 42% had ISS stage II, 38% had ISS stage III disease and 84% had standard risk cytogenetics. Efficacy was evaluated by PFS based on IMWG criteria and overall survival (OS). With a median follow-up of 16.5 months, the primary analysis of PFS in study MMY3007 showed an improvement in the D-VMP arm as compared to the VMP arm; the median PFS had not been reached in the D-VMP arm and was 18.1 months in the VMP arm (HR=0.5; 95% CI: 0.38, 0.65; p<0.0001). Results of an updated PFS analysis after a median follow-up of 40 months continued to show an improvement in PFS for patients in the D-VMP arm compared with the VMP arm. Median PFS was 36.4 months in the D-VMP arm and 19.3 months in the VMP arm (HR=0.42; 95% CI: 0.34, 0.51; p<0.0001), representing a 58% reduction in the risk of disease progression or death in patients treated with D-VMP. Figure 3: Kaplan-Meier curve of PFS in study MMY3007 After a median follow-up of 40 months, D-VMP has shown an OS advantage over the VMP arm (HR=0.60; 95% CI: 0.46, 0.80; p=0.0003), representing a 40% reduction in the risk of death in patients treated in the D-VMP arm. After a median follow-up of 87 months, the median OS was 83 months (95% CI: 72.5, NE) in the D-VMP arm and 53.6 months (95% CI: 46.3, 60.9) in the VMP arm . Figure 4: Kaplan-Meier curve of OS in study MMY3007 Additional efficacy results from study MMY3007 are presented in table 9 below. Table 9: Additional efficacy results from study MMY3007a D-VMP (n=350) VMP (n=356) Overall response (sCR+CR+VGPR+PR) [n(%)] 318 (90.9) 263 (73.9) p-valueb <0.0001 Stringent complete response (sCR) [n(%)] 63 (18.0) 25 (7.0) Complete response (CR) [n(%)] 86 (24.6) 62 (17.4) Very good partial response (VGPR) [n(%)] 100 (28.6) 90 (25.3) Partial response (PR) [n(%)] 69 (19.7) 86 (24.2) MRD negativity rate (95% CI) c (%) 22.3 (18.0, 27.0) 6.2 (3.9, 9.2) Odds ratio with 95% CI d 4.36 (2.64, 7.21) p-valuee <0.0001 D-VMP=daratumumab-bortezomib-melphalan-prednisone; VMP=bortezomib-melphalan-prednisone; MRD=minimal residual disease; CI=confidence interval a Based on intent-to-treat population b p-value from Cochran Mantel-Haenszel Chi-Squared test. c Based on threshold of 10-5 d A Mantel-Haenszel estimate of the common odds ratio for stratified tables is used. An odds ratio >1 indicates an advantage for D-VMP. e p-value from Fisherʼs exact test. In responders, the median time to response was 0.79 months (range: 0.4 to 15.5 months) in the D-VMP group and 0.82 months (range: 0.7 to 12.6 months) in the VMP group. The median duration of response had not been reached in the D-VMP group and was 21.3 months (range: 18.4, not estimable) in the VMP group. A subgroup analysis was performed on patients at least 70 years old, or those 65-69 years old with ECOG performance score of 2, or aged less than 65 years of age with significant comorbidity or ECOG performance score of 2 (D-VMP: n=273, VMP: n=270). The efficacy results in this subgroup were consistent with the overall population. In this subgroup, median PFS was not reached in the D-VMP group and was 17.9 months in the VMP group (HR=0.56; 95% CI: 0.42, 0.75; p<0.0001). The overall response rate was 90% in the D-VMP group and 74% in theVMP group (VGPR rate:29% in D-VMP group and 26% in VMP group; CR: 22% in D-VMP group and 18% in VMP group; sCR rate: 20% in D-VMP group and 7% in VMP group). The safety results of this subgroup were consistent with the overall population. Furthermore, safety analysis of the subgroup of patients with an ECOG performance score of 2 (D-VMP: n=89, VMP: n=84), was also consistent with the overall population. Combination treatment with bortezomib, thalidomide and dexamethasone (VTd) in patients eligible for autologous stem cell transplant (ASCT): Study MMY3006 is a 2 part, open-label, randomised, active-controlled phase III study. Part 1 compared induction and consolidation treatment with intravenous daratumumab 16 mg/kg in combination with bortezomib, thalidomide and dexamethasone (D-VTd) to treatment with bortezomib, thalidomide and dexamethasone (VTd) in patients with newly diagnosed multiple myeloma eligible for ASCT. The consolidation phase of treatment began a minimum of 30 days post-ASCT, when the patient had recovered sufficiently, and engraftment was complete. In part 2, subjects with at least a partial response (PR) by day 100 post-transplant were re-randomised in a 1:1 ratio to daratumumab maintenance or observation only. Only results from part 1 are described henceforth. Bortezomib was administered by subcutaneous injection or intravenous injection at a dose of 1.3 mg/m2 body surface area twice weekly for two weeks (days 1, 4, 8, and 11) of repeated 28 day (4-week) induction treatment cycles (cycles 1-4) and two consolidation cycles (cycles 5 and 6) following ASCT after cycle 4. Thalidomide was administered orally at 100 mg daily during the six bortezomib cycles. Dexamethasone (oral or intravenous) was administered at 40 mg on days 1, 2, 8, 9, 15, 16, 22 and 23 of cycles 1 and 2, and at 40 mg on days 1-2 and 20 mg on subsequent dosing days (days 8, 9, 15, 16) of cycles 3-4. Dexamethasone 20 mg was administered on days 1, 2, 8, 9, 15, 16 in cycles 5 and 6. On the days of intravenous daratumumab infusion, the dexamethasone dose was administered intravenously as a pre-infusion medicinal product. Dose adjustments for bortezomib, thalidomide and dexamethasone were applied according to manufacturer’s prescribing information. A total of 1085 patients were randomised: 543 to the D-VTd arm and 542 to the VTd arm. The baseline demographic and disease characteristics were similar between the two treatment groups. The median age was 58 (range: 22 to 65) years. All patients were ≤65 years: 43% were in the age group ≥60-65 years, 41% were in the age group ≥50-60 years and 16% below age of 50 years. The majority were male (59%), 48% had an ECOG performance score of 0, 42% had an ECOG performance score of 1 and 10% had an ECOG performance score of 2. Forty percent had International Staging System (ISS) stage I, 45% had ISS stage II and 15% had ISS stage III disease. Efficacy was evaluated by the stringent complete response (sCR) rate at day 100 post-transplant and PFS. Table 10: Efficacy results from study MMY3006a D-VTd (n=543) VTd (n=542) P valueb Response assessment day 100 post-transplant Stringent complete response (sCR) 157 (28.9%) 110 (20.3%) 0.0010 CR or better (sCR+CR) 211 (38.9%) 141 (26.0%) <0.0001 Very good partial response or better (sCR+CR+VGPR) 453 (83.4%) 423 (78.0%) MRD negativityc, d n(%) 346 (63.7%) 236 (43.5%) <0.0001 95% CI (%) (59.5%, 67.8%) (39.3%, 47.8%) Odds ratio with 95% CIe 2.27 (1.78, 2.90) MRD negativity in combination with CR or 183 (33.7%) 108 (19.9%) <0.0001 betterc n(%) 95% CI (%) (29.7%, 37.9%) (16.6%, 23.5%) Odds ratio with 95% CIe 2.06 (1.56, 2.72) D-VTd=daratumumab-bortezomib-thalidomide-dexamethasone; VTd=bortezomib-thalidomide-dexamethasone; MRD=minimal residual disease; CI=confidence interval a Based on intent-to-treat population b p-value from Cochran Mantel-Haenszel Chi-Squared test. c Based on threshold of 10-5 d Regardless of response per IMWG e Mantel-Haenszel estimate of the common odds ratio for stratified tables is used. With a median follow-up of 18.8 months, the primary analysis of PFS by censoring patients who were randomised to daratumumab maintenance in the second randomisation at the date of the second randomisation showed HR=0.50; 95% CI: 0.34, 0.75; p=0.0005. Results of an updated PFS analysis with a median follow-up of 44.5 months, censoring patients who were randomised to daratumumab maintenance in the second randomisation, showed HR=0.43; 95% CI: 0.33, 0.55; p<0.0001. Median PFS was not reached in the D-VTd arm and was 37.8 months in the VTd arm. Figure 5: Kaplan-Meier curve of PFS in study MMY3006 Relapsed/refractory multiple myeloma Monotherapy: The clinical efficacy and safety of intravenous daratumumab monotherapy for the treatment of adult patients with relapsed and refractory multiple myeloma whose prior therapy included a proteasome inhibitor and an immunomodulatory agent and who had demonstrated disease progression on the last therapy, was demonstrated in two open-label studies. In study MMY2002, 106 patients with relapsed and refractory multiple myeloma received 16 mg/kg intravenous daratumumab until disease progression. The median patient age was 63.5 years (range, 31 to 84 years), 11% of patients were ≥75 years of age, 49% were male and 79% were Caucasian. Patients had received a median of 5 prior lines of therapy. Eighty percent of patients had received prior autologous stem cell transplantation (ASCT). Prior therapies included bortezomib (99%), lenalidomide (99%), pomalidomide (63%) and carfilzomib (50%). At baseline, 97% of patients were refractory to the last line of treatment, 95% were refractory to both, a proteasome inhibitor (PI) and immunomodulatory agent (IMiD), 77% were refractory to alkylating agents, 63% were refractory to pomalidomide and 48% of patients were refractory to carfilzomib. Efficacy results of the pre-planned interim analysis based on Independent Review Committee (IRC) assessment are presented in table 11 below. Table 11: IRC assessed efficacy results for study MMY2002 Efficacy endpoint Intravenous daratumumab 16 mg/kg N=106 Overall response rate1 (ORR: sCR+CR+VGPR+PR) [n (%)] 31 (29.2) 95% CI (%) (20.8, 38.9) Stringent complete response (sCR) [n (%)] 3 (2.8) Complete response (CR) [n] 0 Very good partial response (VGPR) [n (%)] 10 (9.4) Partial response (PR) [n (%)] 18 (17.0) Clinical benefit rate (ORR+MR) [n (%)] 36 (34.0) Median duration of response [months (95% CI)] 7.4 (5.5, NE) Median time to response [months (range)] 1 (0.9; 5.6) 1 Primary efficacy endpoint (International Myeloma Working Group criteria) CI=confidence interval; NE=not estimable; MR=minimal response Overall response rate (ORR) in MMY2002 was similar regardless of type of prior anti-myeloma therapy. At a survival update with a median duration of follow-up of 14.7 months, median OS was 17.5 months (95% CI:13.7, not estimable). In study GEN501, 42 patients with relapsed and refractory multiple myeloma received 16 mg/kg intravenous daratumumab until disease progression. The median patient age was 64 years (range, 44 to 76 years), 64% were male and 76% were Caucasian. Patients in the study had received a median of 4 prior lines of therapy. Seventy-four percent of patients had received prior ASCT. Prior therapies included bortezomib (100%), lenalidomide (95%), pomalidomide (36%) and carfilzomib (19%). At baseline, 76% of patients were refractory to the last line of treatment, 64% were refractory to both a PI and IMiD, 60% were refractory to alkylating agents, 36% were refractory to pomalidomide and 17% were refractory to carfilzomib. Pre-planned interim analysis showed that treatment with daratumumab at 16 mg/kg led to a 36% ORR with 5% CR and 5% VGPR. The median time to response was 1 (range: 0.5 to 3.2) month. The median duration of response was not reached (95% CI: 5.6 months, not estimable). At a survival update with a median duration of follow-up of 15.2 months, median OS was not reached (95% CI: 19.9 months, not estimable), with 74% of subjects still alive. Combination treatment with lenalidomide: Study MMY3003, an open-label, randomised, active-controlled phase III study, compared treatment with intravenous daratumumab 16 mg/kg in combination with lenalidomide and low-dose dexamethasone (DRd) to treatment with lenalidomide and low-dose dexamethasone (Rd) in patients with relapsed or refractory multiple myeloma who had received at least one prior therapy. Lenalidomide (25 mg once daily orally on days 1-21 of repeated 28-day [4-week] cycles) was given with low dose dexamethasone at 40 mg/week (or a reduced dose of 20 mg/week for patients >75 years or BMI <18.5). On intravenous daratumumab infusion days, 20 mg of the dexamethasone dose was given as a pre-infusion medicinal product and the remainder given the day after the infusion. Treatment was continued in both arms until disease progression or unacceptable toxicity. A total of 569 patients were randomised; 286 to the DRd arm and 283 to the Rd arm. The baseline demographic and disease characteristics were similar between the intravenous daratumumab and the control arm. The median patient age was 65 years (range 34 to 89 years) and 11% were ≥75 years. The majority of patients (86%) received a prior PI, 55% of patients had received a prior IMiD, including 18% of patients who had received prior lenalidomide; and 44% of patients had received both a prior PI and IMiD. At baseline, 27% of patients were refractory to the last line of treatment. Eighteen percent (18%) of patients were refractory to a PI only, and 21% were refractory to bortezomib. Patients refractory to lenalidomide were excluded from the study. With a median follow-up of 13.5 months, the primary analysis of PFS in study MMY3003 demonstrated an improvement in the DRd arm as compared to the Rd arm; the median PFS had not been reached in the DRd arm and was 18.4 months in the Rd arm (HR=0.37; 95% CI: 0.27, 0.52; p<0.0001). Results of an updated PFS analysis after a median follow-up of 55 months continued to show an improvement in PFS for patients in the DRd arm compared with the Rd arm. Median PFS was 45.0 months in the DRd arm and 17.5 months in the Rd arm (HR=0.44; 95% CI: 0.35, 0.54; p<0.0001), representing a 56% reduction in the risk of disease progression or death in patients treated with DRd (see figure 6). Figure 6: Kaplan-Meier curve of PFS in study MMY3003 After a median follow-up of 80 months, DRd has shown an OS advantage over the Rd arm (HR=0.73; 95% CI: 0.58, 0.91; p=0.0044).. The median OS was 67.6 months in the DRd arm and 51.8 months in the Rd arm. Figure 7: Kaplan-Meier curve of OS in study MMY3003 Additional efficacy results from study MMY3003 are presented in table 12 below. Table 12: Additional efficacy results from study MMY3003 Response evaluable patient number DRd (n=281) Rd (n=276) Overall response (sCR+CR+VGPR+PR) n(%) 261 (92.9) 211 (76.4) p-valuea <0.0001 Stringent complete response (sCR) 51 (18.1) 20 (7.2) Complete response (CR) 70 (24.9) 33 (12.0) Very good partial response (VGPR) 92 (32.7) 69 (25.0) Partial response (PR) 48 (17.1) 89 (32.2) Median Time to Response [months (95% CI)] 1.0 (1.0, 1.1) 1.3 (1.1, 1.9) Median Duration of Response [months (95% NE (NE, NE) 17.4 (17.4, NE) CI)] MRD negative rate (95% CI) b (%) 21.0 (16.4, 26.2) 2.8 (1.2, 5.5) Odds ratio with 95% CIc 9.31 (4.31, 20.09) P-valued <0.0001 DRd=daratumumab-lenalidomide-dexamethasone; Rd=lenalidomide-dexamethasone; MRD=minimal residual disease; CI=confidence interval; NE=not estimable. a p-value from Cochran Mantel-Haenszel Chi-Squared test. b Based on Intent-to-treat population and threshold of 10-5 c Mantel-Haenszel estimate of the common odds ratio is used. An odds ratio >1 indicates an advantage for DRd. d p-value is from a Fisher’s exact test. Combination treatment with bortezomib: Study MMY3004, an open-label, randomised, active-controlled phase III study, compared treatment with intravenous daratumumab 16 mg/kg in combination with bortezomib and dexamethasone (DVd), to treatment with bortezomib and dexamethasone (Vd) in patients with relapsed or refractory multiple myeloma who had received at least one prior therapy. Bortezomib was administered by subcutaneous injection or intravenous injection at a dose of 1.3 mg/m2 body surface area twice weekly for two weeks (days 1, 4, 8, and 11) of repeated 21 day (3-week) treatment cycles, for a total of 8 cycles. Dexamethasone was administered orally at a dose of 20 mg on days 1, 2, 4, 5, 8, 9, 11, and 12 of each of the 8 bortezomib cycles (80 mg/week for two out of three weeks of the bortezomib cycle) or a reduced dose of 20 mg/week for patients >75 years, BMI <18.5, poorly controlled diabetes mellitus or prior intolerance to steroid therapy. On the days of intravenous daratumumab infusion, 20 mg of the dexamethasone dose was administered as a pre-infusion medicinal product. intravenous daratumumab treatment was continued until disease progression or unacceptable toxicity. A total of 498 patients were randomised; 251 to the DVd arm and 247 to the Vd arm. The baseline demographic and disease characteristics were similar between the intravenous daratumumab and the control arm. The median patient age was 64 years (range 30 to 88 years) and 12% were ≥75 years. Sixty-nine percent (69%) of patients had received a prior PI (66% received bortezomib) and 76% of patients received an IMiD (42% received lenalidomide). At baseline, 32% of patients were refractory to the last line of treatment. Thirty-three percent (33%) of patients were refractory to an IMiD only, and 28% were refractory to lenalidomide. Patients refractory to bortezomib were excluded from the study. With a median follow-up of 7.4 months, the primary analysis of PFS in study MMY3004 demonstrated an improvement in the DVd arm as compared to the Vd arm; the median PFS had not been reached in the DVd arm and was 7.2 months in the Vd arm (HR [95% CI]: 0.39 [0.28, 0.53]; p-value<0.0001). Results of an updated PFS analysis after a median follow-up of 50 months continued to show an improvement in PFS for patients in the DVd arm compared with the Vd arm. Median PFS was 16.7 months in the DVd arm and 7.1 months in the Vd arm (HR [95% CI]: 0.31 [0.24, 0.39]; p-value<0.0001), representing a 69% reduction in the risk of disease progression or death in patients treated with DVd versus Vd (see figure 8). Figure 8: Kaplan-Meier curve of PFS in study MMY3004 After a median follow-up of 73 months, DVd has shown an OS advantage over the Vd arm (HR=0.74; 95% CI: 0.59, 0.92; p=0.0075). The median OS was 49.6 months in the DVd arm and 38.5 months in the Vd arm. Figure 9: Kaplan-Meier curve of OS in study MMY3004 Additional efficacy results from study MMY3004 are presented in table 13 below. Table 13: Additional efficacy results from study MMY3004 Response evaluable patient number DVd (n=240) Vd (n=234) Overall response (sCR+CR+VGPR+PR) n(%) 199 (82.9) 148 (63.2) P-value a <0.0001 Stringent complete response (sCR) 11 (4.6) 5 (2.1) Complete response (CR) 35 (14.6) 16 (6.8) Very good partial response (VGPR) 96 (40.0) 47 (20.1) Partial response (PR) 57 (23.8) 80 (34.2) Median time to response [months (range)] 0.9 (0.8, 1.4) 1.6 (1.5, 2.1) Median duration of response [months (95% CI)] NE (11.5, NE) 7.9 (6.7, 11.3) MRD negative rate (95% CI)b 8.8% (5.6%, 13.0%) 1.2% (0.3%, 3.5%) Odds ratio with 95% CIc 9.04 (2.53, 32.21) P-valued 0.0001 DVd=daratumumab- bortezomib-dexamethasone; Vd=bortezomib-dexamethasone; MRD=minimal residual disease; CI=confidence interval; NE=not estimable. a p-value from Cochran Mantel-Haenszel Chi-Squared test. b Based on Intent-to-treat population and threshold of 10-5 c Mantel-Haenszel estimate of the common odds ratio is used. An odds ratio >1 indicates an advantage for DVd. d p-value is from Fisher’s exact test. Cardiac electrophysiology Daratumumab as a large protein has a low likelihood of direct ion channel interactions. The effect of daratumumab on the QTc interval was evaluated in an open-label study for 83 patients (Study GEN501) with relapsed and refractory multiple myeloma following daratumumab infusions (4 to 24 mg/kg). Linear mixed PK-PD analyses indicated no large increase in mean QTcF interval (i.e. greater than 20 ms) at daratumumab Cmax.
Pharmacokinetic Properties
5.2 Pharmacokinetic properties Daratumumab exposure in a monotherapy study following the recommended 1,800 mg administration of DARZALEX 120MG/ML S.C. 1,800MG subcutaneous formulation (weekly for 8 weeks, biweekly for 16 weeks, monthly thereafter) as compared to 16 mg/kg intravenous daratumumab for the same dosing schedule, showed non-inferiority for the co-primary endpoint of maximum Ctrough (cycle 3 day 1 pre-dose), with mean ± SD of 593 ± 306 µg/mL compared to 522 ± 226 µg/mL for intravenous daratumumab, with a geometric mean ratio of 107.93% (90% CI: 95.74-121.67). Following the recommended dose of 1,800 mg DARZALEX 120MG/ML S.C. 1,800MG solution for subcutaneous injection, peak concentrations (Cmax) increased 4.8-fold and total exposure (AUC0-7 days) increased 5.4-fold from first dose to last weekly dose (8th dose). Highest trough concentrations for DARZALEX 120MG/ML S.C. 1,800MG solution for subcutaneous injection are typically observed at the end of the weekly dosing regimens for both monotherapy and combination therapy. The simulated trough concentrations following 6 weekly doses of 1,800 mg DARZALEX 120MG/ML S.C. 1,800MG solution for subcutaneous injection for combination therapy were similar to 1,800 mg DARZALEX 120MG/ML S.C. 1,800MG solution for subcutaneous injection monotherapy. Absorption and distribution At the recommended dose of 1,800 mg, the absolute bioavailability of DARZALEX 120MG/ML S.C. 1,800MG solution for subcutaneous injection is 69%, with an absorption rate of 0.012 hour-1, with peak concentrations occurring at 70 to 72 h (Tmax). The model predicted mean estimate of the volume of distribution for the central compartment was 5.25 L (36.9% CV) and peripheral compartment was 3.78 L, suggesting that daratumumab is primarily localised to the vascular system with limited extravascular tissue distribution. Metabolism and elimination Daratumumab exhibits both concentration and time-dependent pharmacokinetics with parallel linear and nonlinear (saturable) elimination that is characteristic of target-mediated clearance. The population PK model estimated mean clearance value of daratumumab is 4.96 mL/h (58.7% CV). The model-based geometric mean for half-life associated with linear elimination is 20.4 days (22.4% CV). For the monotherapy regimen, the steady state is achieved at approximately 5 months into every 4 weeks dosage at the recommended dose and schedule (1,800 mg; once weekly for 8 weeks, every 2 weeks for 16 weeks, and then every 4 weeks thereafter). A population PK analysis was conducted using data from DARZALEX 120MG/ML S.C. 1,800MG solution for subcutaneous injection monotherapy and combination therapy, and the predicted PK exposures are summarised in table 14. Table 14: Daratumumab exposure following administration of DARZALEX 120MG/ML S.C. 1,800MG subcutaneous formulation (1,800 mg) or intravenous daratumumab (16 mg/kg) monotherapy PK parameters Cycles subcutaneous intravenous daratumumab daratumumab Median (5th; 95th Median (5th; 95th percentile) percentile) Cycle 1, 1st weekly dose 123 (36; 220) 112 (43; 168) Ctrough (µg/mL) Cycle 2, last weekly dose 563 (177; 1063) 472 (144; 809) (cycle 3 day 1 Ctrough) Cycle 1, 1st weekly dose 132 (54; 228) 256 (173; 327) Cmax (µg/mL) Cycle 2, last weekly dose 592 (234; 1114) 688 (369; 1061) Cycle 1, 1st weekly dose 720 (293; 1274) 1187 (773; 1619) AUC0-7 days (µg/mL•day) Cycle 2, last weekly dose 4017 (1515; 7564) 4019 (1740; 6370) Special populations Age and gender Based on population PK analyses in patients (33-92 years) receiving monotherapy or various combination therapies, age had no statistically significant effect on the PK of daratumumab. No individualisation is necessary for patients on the basis of age. Gender had a statistically significant effect on PK, with slightly higher exposure in females than males, but the difference in exposure is not considered clinically meaningful. No individualisation is necessary for patients on the basis of gender. Renal impairment No formal studies of DARZALEX 120MG/ML S.C. 1,800MG subcutaneous formulation in patients with renal impairment have been conducted. Population PK analyses were performed based on pre-existing renal function data in patients receiving DARZALEX 120MG/ML S.C. 1,800MG subcutaneous formulation monotherapy or various combination therapies in patients with multiple myeloma. No clinically important differences in exposure to daratumumab were observed between patients with renal impairment and those with normal renal function. Hepatic impairment No formal studies of DARZALEX 120MG/ML S.C. 1,800MG subcutaneous formulation in patients with hepatic impairment have been conducted. Population PK analyses were performed in patients receiving DARZALEX 120MG/ML S.C. 1,800MG subcutaneous formulation monotherapy or various combination therapies in patients with multiple myeloma. No clinically important differences in the exposure to daratumumab were observed between patients with normal hepatic function and mild hepatic impairment. There were very few patients with moderate and severe hepatic impairment to make meaningful conclusions for these populations. Race Based on the population PK analyses in patients receiving either DARZALEX 120MG/ML S.C. 1,800MG subcutaneous formulation monotherapy or various combination therapies, the daratumumab exposure was similar across races. Body weight The flat-dose administration of DARZALEX 120MG/ML S.C. 1,800MG subcutaneous formulation 1,800 mg as monotherapy achieved adequate exposure for all body-weight subgroups. The mean cycle 3 day 1 Ctrough in the lower body-weight subgroup (≤65 kg) was 60% higher and in the higher body weight (>85 kg) subgroup, 12% lower than the intravenous daratumumab subgroup. In some patients with body weight >120 kg, lower exposure was observed which may result in reduced efficacy. However, this observation is based on limited number of patients.

פרטי מסגרת הכללה בסל
א. התרופה תינתן לטיפול במקרים האלה: 1. מיאלומה נפוצה כקו טיפול ראשון:א. בשילוב עם Thalidomide, Bortezomib ו-Dexamethasone עבור מטופלים המועמדים להשתלת תאי גזע. הטיפול במשלב זה לא יינתן כטיפול אחזקה לאחר השתלת תאי גזע.ב. בשילוב עם Bortezomib, Melphalan ו-Prednisone עבור מטופלים שאינם מועמדים להשתלת תאי גזע. ג. בשילוב עם Lenalidomide ו-Dexamethasone עבור מטופלים שאינם מועמדים להשתלת תאי גזע. 2. מיאלומה נפוצה כקו טיפול שני:א. בשילוב עם Lenalidomide ו-Dexamethasone בחולה שמחלתו התקדמה לאחר טיפול קודם במשלב שכלל Thalidomide או Bortezomib ולא כלל Lenalidomide.ב. בשילוב עם Bortezomib ו-Dexamethasone בחולה שמחלתו התקדמה לאחר טיפול קודם במשלב שכלל Lenalidomide.3. כמונותרפיה בקו טיפול רביעי והלאה לטיפול במיאלומה נפוצה בחולה שמחלתו עמידה או נשנית לאחר מיצוי טיפול בתרופות ממשפחת מעכבי פרוטאזום ותרופות ממשפחת התכשירים האימונומודולטוריים. ב. הטיפול בתרופה יינתן לחולה שטרם טופל ב-Daratumumab למחלתו. ג. החולה יהיה זכאי במהלך מחלתו לטיפול בתרופה אחת בלבד מהתרופות המפורטות להלן - Daratumumab, Elotuzumab, Ixazomib. אולם אם טופל בקווים מוקדמים של המחלה באחת מהתרופות הבאות – Carfilzomib, Elotuzumab, Ixazomib – ולא טופל ב-Daratumumab לא יהיה בכך כדי למנוע מהחולה קבלת הטיפול ב-Daratumumab בקו רביעי בהתאם לסעיף (3) דלעיל.ד. מתן התרופה האמורה ייעשה לפי מרשם של מומחה באונקולוגיה או מומחה בהמטולוגיה.
מסגרת הכללה בסל
התוויות הכלולות במסגרת הסל
| התוויה | תאריך הכללה | תחום קליני | Class Effect | מצב מחלה |
|---|---|---|---|---|
| מיאלומה נפוצה כקו טיפול ראשון: א. בשילוב עם Thalidomide, Bortezomib ו-Dexamethasone עבור מטופלים המועמדים להשתלת תאי גזע. הטיפול במשלב זה לא יינתן כטיפול אחזקה לאחר השתלת תאי גזע. ב. בשילוב עם Bortezomib, Melphalan ו-Prednisone עבור מטופלים שאינם מועמדים להשתלת תאי גזע. ג. בשילוב עם Lenalidomide ו-Dexamethasone עבור מטופלים שאינם מועמדים להשתלת תאי גזע. ב. הטיפול בתרופה יינתן לחולה שטרם טופל ב-Daratumumab למחלתו. ג. החולה יהיה זכאי במהלך מחלתו לטיפול בתרופה אחת בלבד מהתרופות המפורטות להלן - Daratumumab, Elotuzumab, Ixazomib. אולם אם טופל בקווים מוקדמים של המחלה באחת מהתרופות הבאות – Carfilzomib, Elotuzumab, Ixazomib – ולא טופל ב-Daratumumab לא יהיה בכך כדי למנוע מהחולה קבלת הטיפול ב-Daratumumab בקו רביעי בהתאם לסעיף (3) דלעיל. ד. מתן התרופה האמורה ייעשה לפי מרשם של מומחה באונקולוגיה או מומחה בהמטולוגיה. | 01/02/2023 | אונקולוגיה | מיאלומה נפוצה, Multiple myeloma | |
| מיאלומה נפוצה כקו טיפול שני: א. בשילוב עם Lenalidomide ו-Dexamethasone בחולה שמחלתו התקדמה לאחר טיפול קודם במשלב שכלל Thalidomide או Bortezomib ולא כלל Lenalidomide. ב. בשילוב עם Bortezomib ו-Dexamethasone בחולה שמחלתו התקדמה לאחר טיפול קודם במשלב שכלל Lenalidomide. החולה יהיה זכאי במהלך מחלתו לטיפול בתרופה אחת בלבד מהתרופות המפורטות להלן - Carfilzomib, Daratumumab, Elotuzumab, Ixazomib. אולם אם טופל באחת מהתרופות האלה – Carfilzomib, Elotuzumab, Ixazomib - לא יהיה בכך כדי למנוע מהחולה קבלת הטיפול ב-Daratumumab בקו רביעי בהתאם לסעיף (2) להלן. | 16/01/2019 | אונקולוגיה | מיאלומה נפוצה, multiple myeloma | |
| מיאלומה נפוצה כקו טיפול שני בשילוב עם Lenalidomide ו-Dexamethasone בחולה שמחלתו התקדמה לאחר טיפול קודם במשלב שכלל Thalidomide או Bortezomib ולא כלל Lenalidomide. בחולה המוגדר בסיכון גבוה יהיה החולה זכאי במהלך מחלתו לטיפול בתרופה אחת בלבד מהתרופות המפורטות להלן - Carfilzomib, Daratumumab, Elotuzumab, Ixazomib. אולם אם טופל באחת מהתרופות האלה – Carfilzomib, Elotuzumab, Ixazomib - לא יהיה בכך כדי למנוע מהחולה קבלת הטיפול ב-Daratumumab בקו רביעי בהתאם לסעיף ב להלן. סיכון גבוה לעניין זה יוגדר בחולה העונה על אחד מאלה: •ציטוגנטיקה מסוג t(4,14) או del 17 p; •חזרה מהירה (תוך פחות מ-12 חודשים) של המחלה לאחר הטיפול הראשוני; •עמידות לטיפול הראשוני | 11/01/2018 | אונקולוגיה | מיאלומה נפוצה, multiple myeloma | |
| כמונותרפיה בקו טיפול רביעי והלאה לטיפול במיאלומה נפוצה בחולה שמחלתו עמידה או נשנית לאחר מיצוי טיפול בתרופות ממשפחת מעכבי פרוטאזום ותרופות ממשפחת התכשירים האימונומודולטוריים. | 12/01/2017 | אונקולוגיה | מיאלומה נפוצה, Multiple myeloma |
שימוש לפי פנקס קופ''ח כללית 1994
לא צוין
תאריך הכללה מקורי בסל
12/01/2017
הגבלות
תרופה מוגבלת לרישום ע'י רופא מומחה או הגבלה אחרת
מידע נוסף
עלון מידע לצרכן
01.02.22 - עלון לצרכן אנגלית 29.03.22 - עלון לצרכן אנגלית 01.02.22 - עלון לצרכן אנגלית 01.02.22 - עלון לצרכן עברית 01.02.22 - עלון לצרכן ערבית 28.11.21 - עלון לצרכן אנגלית 18.10.21 - החמרה לעלון 20.12.21 - החמרה לעלון 12.10.22 - החמרה לעלון 04.01.23 - החמרה לעלון 11.06.23 - החמרה לעלוןלתרופה במאגר משרד הבריאות
דארזלקס 120 מ"ג/מ"ל תת עורי 1,800 מ"ג