Quest for the right Drug
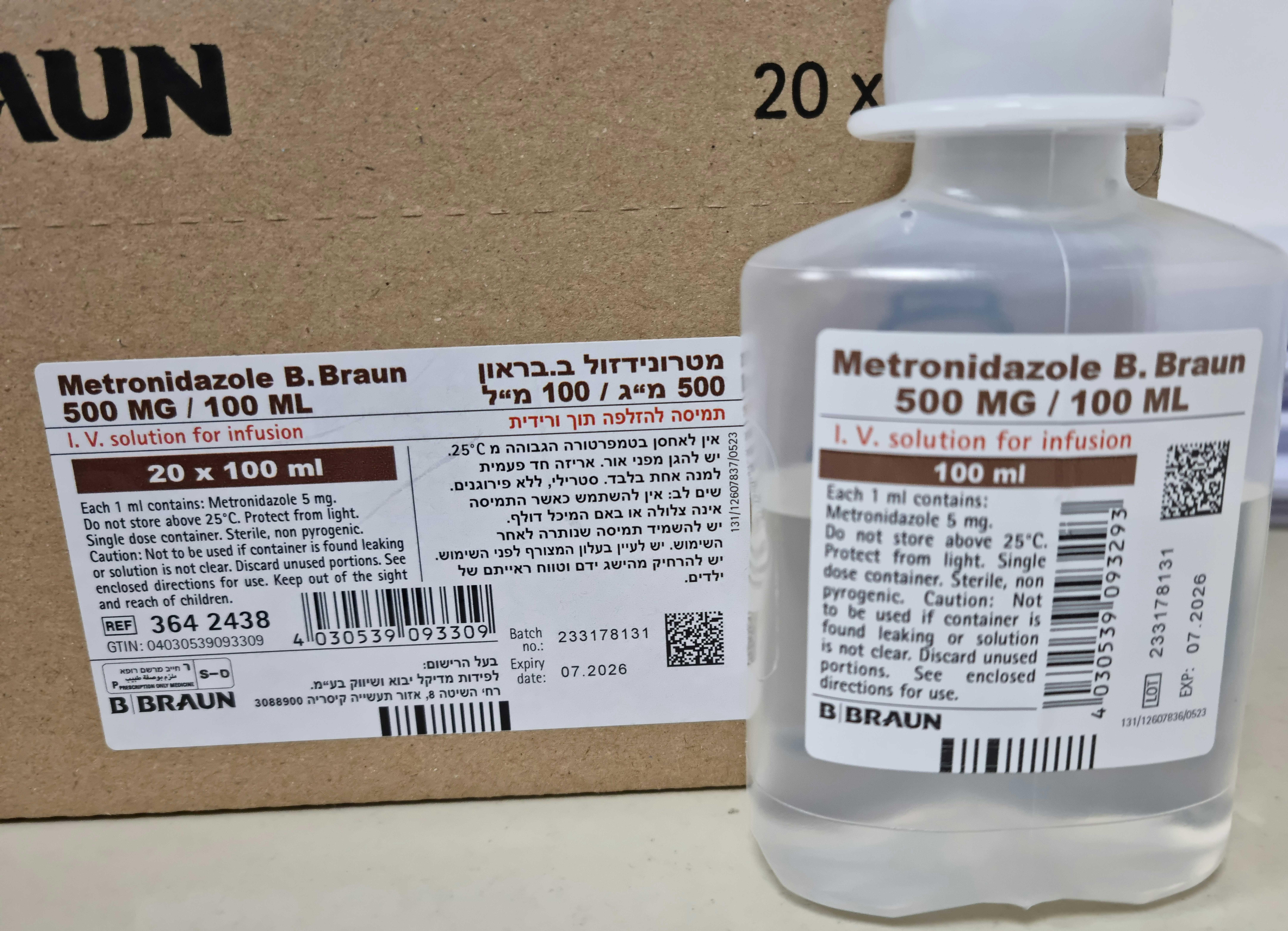
מטרונידזול ב. בראון 500 מ"ג / 100 מ"ל Metronidazole B.Braun 500 MG / 100 ML (METRONIDAZOLE)
תרופה במרשם
תרופה בסל
נרקוטיקה
ציטוטוקסיקה
צורת מתן:
תוך-ורידי : I.V
צורת מינון:
תמיסה לאינפוזיה : SOLUTION FOR INFUSION
עלון לרופא
מינוניםPosology התוויות
Indications תופעות לוואי
Adverse reactions התוויות נגד
Contraindications אינטראקציות
Interactions מינון יתר
Overdose הריון/הנקה
Pregnancy & Lactation אוכלוסיות מיוחדות
Special populations תכונות פרמקולוגיות
Pharmacological properties מידע רוקחי
Pharmaceutical particulars אזהרת שימוש
Special Warning עלון לרופא
Physicians Leaflet
Pharmacological properties : תכונות פרמקולוגיות
Pharmacodynamic Properties
5.1. Pharmacodynamic properties Pharmaco-therapeutic group: Anti-infectives for systemic use – imidazole derivatives ATC Code: J01X D01 Mechanism of action Metronidazole itself is ineffective. It is a stable compound able to penetrate into microorganisms. Under anaerobic conditions nitroso radicals acting on DNA are formed from metronidazole by the microbial pyruvate-ferredoxin-oxidoreductase, with oxidation of ferredoxin and flavodoxin. Nitroso radicals form adducts with base pairs of the DNA, thus leading to breaking of the DNA chain and consecutively to cell death. PK/PD relationship Metronidazole acts in a concentration dependent manner. The efficacy of metronidazole mainly depends on the quotient of the maximum serum concentration (cmax) and the minimum inhibitory concentration (MIC) relevant for the microorganism concerned. Breakpoints For the testing of metronidazole usual dilution series are applied. The following minimum inhibitory concentration have been established to distinguish susceptible from resistant microorganisms: EUCAST (European Committee on Antimicrobial Susceptibility Testing, Version 1.3, January 5, 2011) breakpoints separating susceptible (S) from resistant organisms (R) are as follows: Gram-positive anaerobes (S: ӊ 4 mg/l R > 4 mg/l) Gram-negative anaerobes (S: ӊ 4 mg/l R > 4 mg/l) List of susceptible and resistant organisms. Commonly susceptible species Anaerobes Bacteroides fragilis Clostridium difficile° Clostridium perfringens°Δ Eubacterium Fusobacterium spp.° Peptoniphilus spp.° Peptostreptococcus spp.° Porphyromonas spp.° Prevotella spp. Veillonella spp.° Other micro-organisms Entamoeba histolytica° Gardnerella vaginalis° Giardia lamblia° Trichomonas vaginalis° Inherently resistant organisms All obligate aerobes Gram-positive micro-organisms Actinomyces spp. Enterococcus spp. Propionibacterium acnes Staphylococcus spp. Streptococcus spp. Gram-negative micro-organisms Enterobacteriaceae Haemophilus spp. Mobiluncus ° At the time of publication of these tables, no up-to-date data were available. In primary literature, standard reference books and therapy recommendations susceptibility of the respective strains is assumed. Δ Only to be used in patients with allergy to penicillin Mechanisms of resistance to metronidazole The mechanisms of metronidazole resistance are still understood only in part. Strains of Bacteroides being resistant to metronidazole possess genes encoding nitroimidazole reductases converting nitroimidazoles to aminoimidazoles. Therefore the formation of the antibacterially effective nitroso radicals is inhibited. There is full cross resistance between metronidazole and the other nitroimidazole derivatives (tinidazole, ornidazole, nimorazole). The prevalence of acquired resistance of individual species may vary, depending on region and time. Therefore especially for the adequate treatment of severe infections specific local information regarding resistance should be available. If there is doubt about the efficacy of metronidazole due to the local resistance situation, expert advice should be sought. Especially in the case of severe infections or failure of treatment, microbiological diagnosis including determination of species of the microorganism and its susceptibility to metronidazole is required.
Pharmacokinetic Properties
5.2. Pharmacokinetic properties Absorption: Metronidazole is readily absorbed from the gastrointestinal tract and the oral bioavailability is > 90%. Consequently, the same mg dose will result in similar exposure (AUC) when switching between intravenous and oral dosing. Distribution: Metronidazole is widely distributed in body tissues after injection. It also diffuses across the placenta, and is found in breast milk of nursing mothers in concentrations equivalent to those in serum. Protein binding is less than 20 %, the apparent volume of distribution is 36 litres. Biotransformation: Metronidazole is metabolised in the liver by side-chain oxidation and glucuronide formation. Its metabolites include an acid oxidation product, a hydroxy derivative and glucuronide. The major metabolite in the serum is the hydroxylated metabolite, the major metabolite in the urine is the acid metabolite. Elimination: Approximately 80% of the substance is excreted in urine with less than 10% in the form of the unchanged drug substance . Small quantities are excreted via the liver. Elimination half-life is 8 (6-10) hours. Characteristics in special patient groups: Renal insufficiency delays excretion only to an unimportant degree. The elimination half-life of metronidazole remains unchanged in the presence of renal failure, however such patients retain the metabolites of metronidazole. The clinical significance of this is not known at present. Delayed plasma clearance and prolonged serum half-life (up to 30 h) is to be expected in severe liver disease.

שימוש לפי פנקס קופ''ח כללית 1994
לא צוין
תאריך הכללה מקורי בסל
לא צוין
הגבלות
לא צוין
מידע נוסף
