Quest for the right Drug
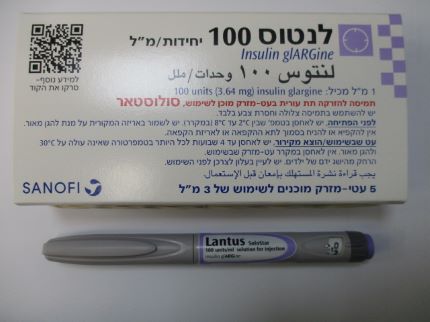
לנטוס 100 יחידות/מ"ל LANTUS 100 UNITS/ML (INSULIN GLARGINE)
תרופה במרשם
תרופה בסל
נרקוטיקה
ציטוטוקסיקה
צורת מתן:
תת-עורי : S.C
צורת מינון:
תמיסה להזרקה : SOLUTION FOR INJECTION
עלון לרופא
מינוניםPosology התוויות
Indications תופעות לוואי
Adverse reactions התוויות נגד
Contraindications אינטראקציות
Interactions מינון יתר
Overdose הריון/הנקה
Pregnancy & Lactation אוכלוסיות מיוחדות
Special populations תכונות פרמקולוגיות
Pharmacological properties מידע רוקחי
Pharmaceutical particulars אזהרת שימוש
Special Warning עלון לרופא
Physicians Leaflet
Adverse reactions : תופעות לוואי
4.8 Undesirable effects Summary of the safety profile Hypoglycaemia (very common), in general the most frequent adverse reaction of insulin therapy, may occur if the insulin dose is too high in relation to the insulin requirement (see section 4.4). Tabulated list of adverse reactions The following related adverse reactions from clinical investigations are listed below by system organ class and in order of decreasing incidence (very common: >1/10; common: >1/100 to <1/10; uncommon: > 1/1,000 to < 1/100; rare: > 1/10,000 to <1/1,000; very rare: < 1/10,000). Within each frequency grouping, adverse reactions are presented in order of decreasing seriousness. MedDRA Very Common Uncommon Rare Very Not known system organ common rare classes Immune Allergic system reactions disorders Metabolism Hypoglycaemia and nutrition disorders Nervous Dysgeusia system disorders Eye disorders Visual impairment, Retinopathy Skin and Lipohypertrophy Lipoatrophy Cutaneous subcutaneous amyloidosis tissue disorders Musculoskeletal Myalgia and connective tissue disorders General Injection site Oedema disorders and reactions administration site conditions Description of selected adverse reactions Metabolism and nutrition disorders Severe hypoglycaemic attacks, especially if recurrent, may lead to neurological damage. Prolonged or severe hypoglycaemic episodes may be life-threatening. In many patients, the signs and symptoms of neuroglycopenia are preceded by signs of adrenergic counter-regulation. Generally, the greater and more rapid the decline in blood glucose, the more marked is the phenomenon of counter-regulation and its symptoms (see section 4.4). Immune system disorders Immediate-type allergic reactions to insulin are rare. Such reactions to insulin (including insulin glargine) or the excipients may, for example, be associated with generalised skin reactions, angiooedema, bronchospasm, hypotension and shock, and may be life-threatening. Eyes disorders A marked change in glycaemic control may cause temporary visual impairment, due to temporary alteration in the turgidity and refractive index of the lens. Long-term improved glycaemic control decreases the risk of progression of diabetic retinopathy. However, intensification of insulin therapy with abrupt improvement in glycaemic control may be associated with temporary worsening of diabetic retinopathy. In patients with proliferative retinopathy, particularly if not treated with photocoagulation, severe hypoglycaemic episodes may result in transient amaurosis. Skin and subcutaneous tissue disorders Lipodystrophy and cutaneous amyloidosis may occur at the injection site and delay local insulin absorption. Continuous rotation of the injection site within the given injection area may help to reduce or prevent these reactions (see section 4.4). General disorders and administration site conditions Injection site reactions include redness, pain, itching, hives, swelling, or inflammation. Most minor reactions to insulins at the injection site usually resolve in a few days to a few weeks. Rarely, insulin may cause sodium retention and oedema particularly if previously poor metabolic control is improved by intensified insulin therapy. Pediatric population In general, the safety profile for children and adolescents (≤ 18 years of age) is similar to the safety profile for adults. The adverse reaction reports received from post marketing surveillance included relatively more frequent injection site reactions (injection site pain, injection site reaction) and skin reactions (rash, urticaria) in children and adolescents (≤ 18 years of age) than in adults. Clinical study safety data are not available for children under 2 years. Reporting of suspected adverse reactions Reporting suspected adverse reactions after authorisation of the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the medicinal product. Any suspected adverse events should be reported to the Ministry of Health according to the National Regulation by using an online form at https://sideeffects.health.gov.il/.

פרטי מסגרת הכללה בסל
התרופה תינתן לחולי סוכרת
מסגרת הכללה בסל
התוויות הכלולות במסגרת הסל
| התוויה | תאריך הכללה | תחום קליני | Class Effect | מצב מחלה |
|---|---|---|---|---|
| INSULIN GLARGINE | ||||
| INSULIN DETEMIR | ||||
| סכרת |
שימוש לפי פנקס קופ''ח כללית 1994
לא צוין
תאריך הכללה מקורי בסל
15/04/2005
הגבלות
תרופה מוגבלת לרישום ע'י רופא מומחה או הגבלה אחרת
מידע נוסף
