Quest for the right Drug
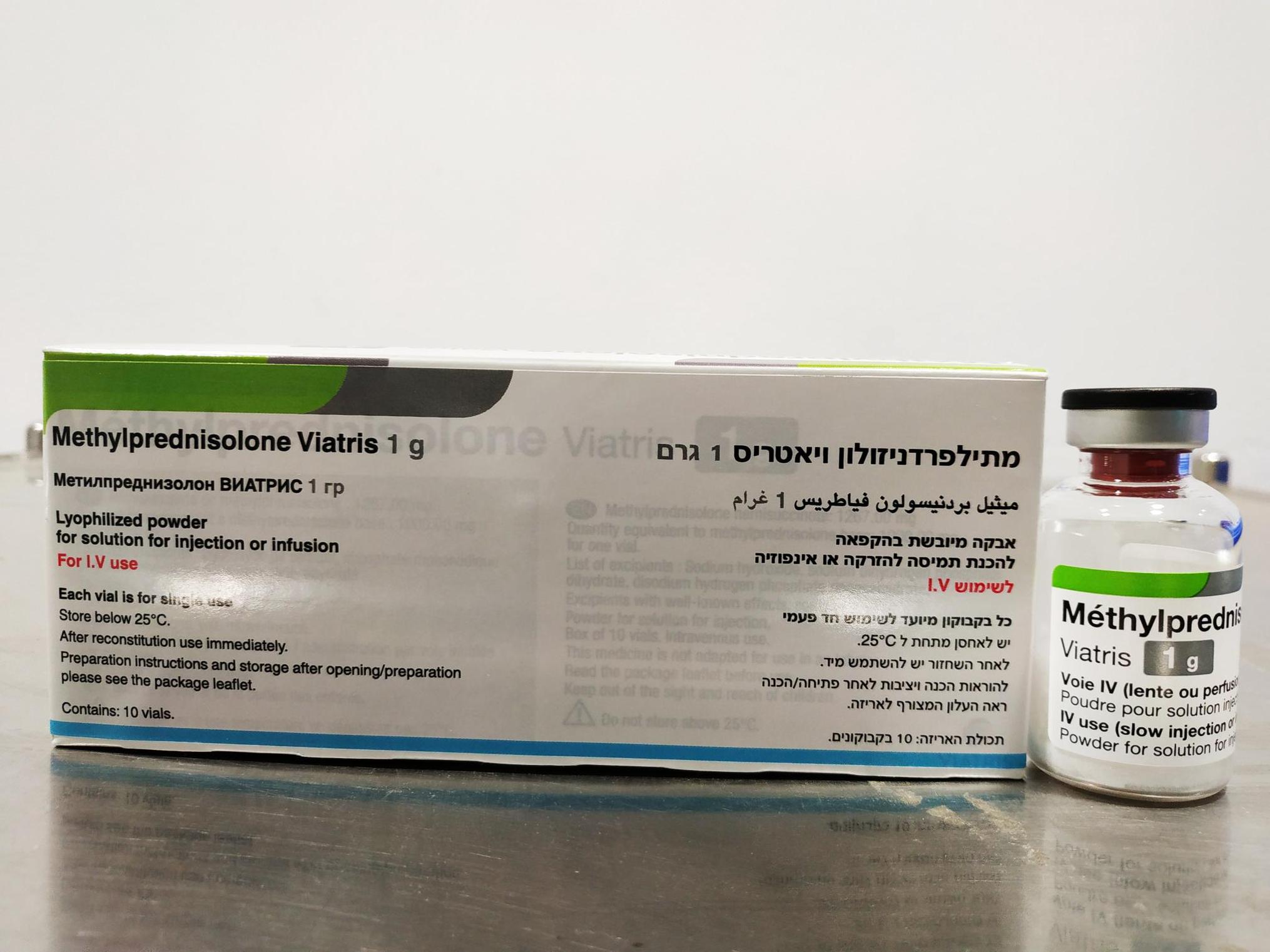
מתילפרדניזולון ויאטריס 1 גרם METHYLPREDNISOLONE VIATRIS 1 G (METHYLPREDNISOLONE AS HEMISUCCINATE)
תרופה במרשם
תרופה בסל
נרקוטיקה
ציטוטוקסיקה
צורת מתן:
תוך-ורידי : I.V
צורת מינון:
אין פרטים : LYOPHILIZED POWDER FOR SOLUTION FOR INJECTION OR INFUSION
עלון לרופא
מינוניםPosology התוויות
Indications תופעות לוואי
Adverse reactions התוויות נגד
Contraindications אינטראקציות
Interactions מינון יתר
Overdose הריון/הנקה
Pregnancy & Lactation אוכלוסיות מיוחדות
Special populations תכונות פרמקולוגיות
Pharmacological properties מידע רוקחי
Pharmaceutical particulars אזהרת שימוש
Special Warning עלון לרופא
Physicians Leaflet
Pharmacological properties : תכונות פרמקולוגיות
Pharmacodynamic Properties
5.1 Pharmacodynamic properties Pharmacotherapeutic group: Glucocorticoids, ATC code: H02AB04 Methylprednisolone is a corticosteroid with an anti-inflammatory activity at least five times that of hydrocortisone. An enhanced separation of glucocorticoid and mineralocorticoid effect results in a reduced incidence of sodium and water retention.
Pharmacokinetic Properties
5.2 Pharmacokinetic properties Methylprednisolone pharmacokinetics is linear, independent of route of administration. Distribution: Methylprednisolone is widely distributed into the tissues, crosses the blood-brain barrier, and is secreted in breast milk. Its apparent volume of distribution is approximately 1.4 L/kg. The plasma protein binding of methylprednisolone in humans is approximately 77%. Biotransformation: Methylprednisolone is extensively bound to plasma proteins, mainly to globulin and less so to albumin. Only unbound corticosteroid has pharmacological effects or is metabolised. Metabolism occurs in the liver and to a lesser extent in the kidney. In humans, methylprednisolone is metabolized in the liver to inactive metabolites; the major ones are 20α- hydroxymethylprednisolone and 20β-hydroxymethylprednisolone. Metabolism in the liver occurs primarily via the CYP3A4. (For a list of drug interactions based on CYP3A4-mediated metabolism, see section 4.5). Methylprednisolone, like many CYP3A4 substrates, may also be a substrate for the ATP- binding cassette (ABC) transport protein p-glycoprotein, influencing tissue distribution and interactions with other medicines. Elimination: Metabolites are excreted in the urine. The mean elimination half-life for total methylprednisolone is in the range of 1.8 to 5.2 hours. Total clearance is approximately 5 to 6 mL/min/kg. Mean elimination half-life ranges from 2.4 to 3.5 hours in normal healthy adults and appears to be independent of the route of administration. Total body clearance following intravenous injection of methylprednisolone to healthy adult volunteers is approximately 15-16 L/hour.

שימוש לפי פנקס קופ''ח כללית 1994
לא צוין
תאריך הכללה מקורי בסל
לא צוין
הגבלות
לא צוין
מידע נוסף
