Quest for the right Drug
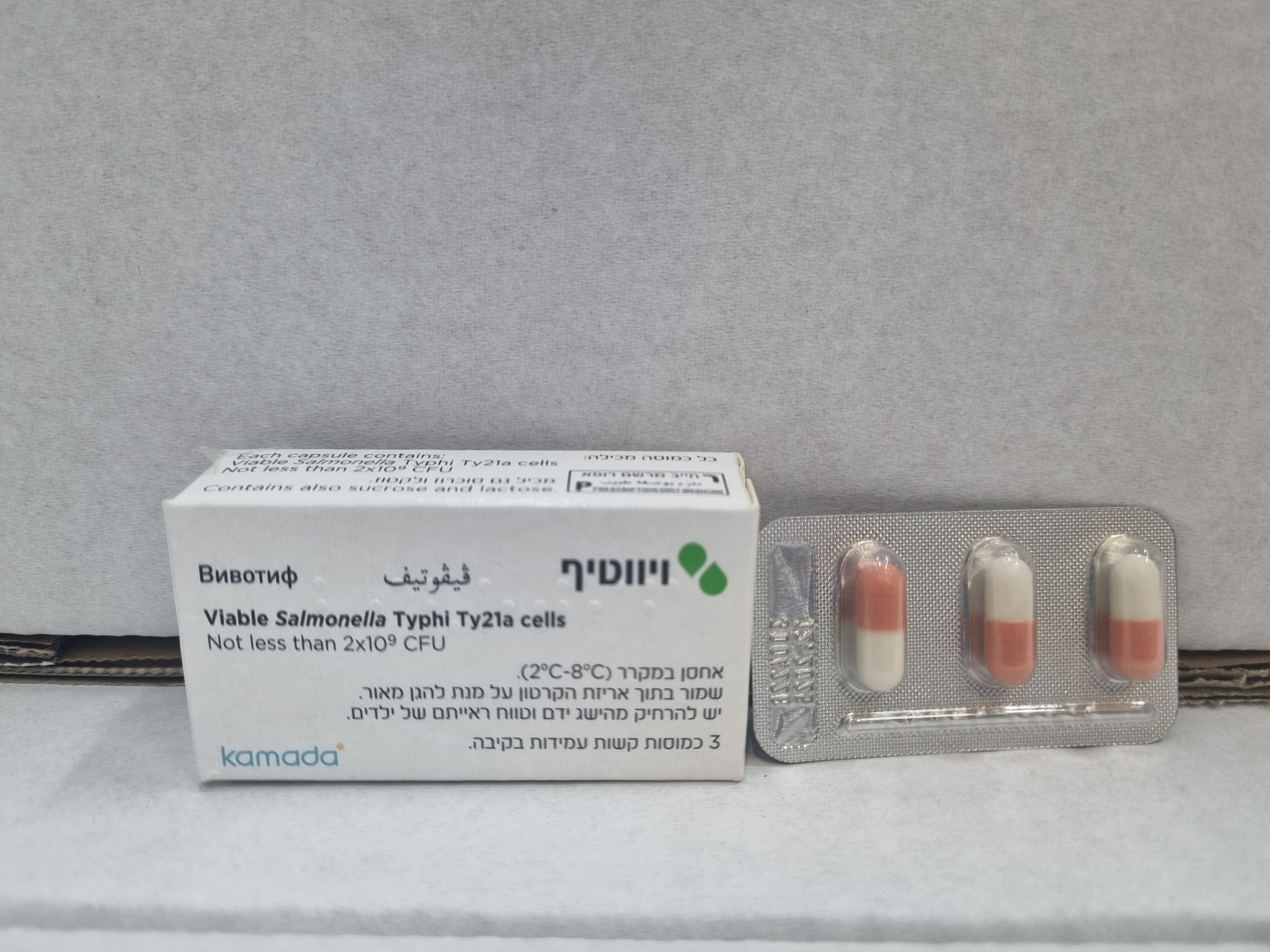
ויווטיף VIVOTIF (VIABLE SALMONELLA TYPHI TY21A CELLS)
תרופה במרשם
תרופה בסל
נרקוטיקה
ציטוטוקסיקה
צורת מתן:
פומי : PER OS
צורת מינון:
אין פרטים : GASTRO RESISTANT HARD CAPSULE
עלון לרופא
מינוניםPosology התוויות
Indications תופעות לוואי
Adverse reactions התוויות נגד
Contraindications אינטראקציות
Interactions מינון יתר
Overdose הריון/הנקה
Pregnancy & Lactation אוכלוסיות מיוחדות
Special populations תכונות פרמקולוגיות
Pharmacological properties מידע רוקחי
Pharmaceutical particulars אזהרת שימוש
Special Warning עלון לרופא
Physicians Leaflet
Pharmacological properties : תכונות פרמקולוגיות
Pharmacodynamic Properties
5.1 Pharmacodynamic properties Pharmacotherapeutic group: Bacterial vaccines, ATC code: J07AP01 Mechanism of action In contrast to virulent S. Typhi which can cause systemic disease, the vaccine strain Ty21a is attenuated as a result of the absence of the Vi capsular polysaccharide virulence factor and the galE mutation which causes irreversible changes in cell wall biosynthesis. The galE mutation limits replication in vivo owing to an accumulation of toxic metabolites, which causes lysis of the bacterial cell. The vaccine strain Ty21a thus remains locally in the intestine and can not be detected systemically or in the stools following ingestion of the usual dose. Ty21a triggers humoral and cellular immunity both locally and systemically. Specifically, Ty21a induces IgA to Salmonella O antigen, as well as antibody-secreting cells (ASCs) and polyfunctional CD4+ and CD8+ T cells with a gut-homing phenotype. IgA and CD8+ responses can be detected in the gastrointestinal tract up to 2 years after Ty21a vaccination. A non-placebo-controlled challenge study in US subjects was conducted with an early formulation and dose regimen of Ty21a which demonstrated 87% protection against virulent S. Typhi following vaccination. Clinical protection against other enteric fever-causing agents including S. Paratyphi has not been shown in randomised, controlled clinical trials. The three-dose regimen of enteric-coated capsules in an every other day schedule has been shown in a field trial to have a protective efficacy of 71% (95% CI 35%-87%) during the first year after vaccination, 67% (95% CI 47%-79%) over three years and 62% (95% CI 48%-73%) protection over seven years of follow-up. Complete vaccination comprises the ingestion of three capsules at Days 1, 3 and 5. The optimal immune response may not be achieved unless the entire vaccination schedule is completed. Two doses were shown to have an efficacy of 59% (95% CI 41%-71%) and one dose had an efficacy of 29% (95% CI 4%-47%) over two years of follow-up. Revaccination studies in healthy volunteers demonstrated that local humoral and cell-mediated immunity induced by the primary vaccination persists for at least three years. The clinical relevance of these observations are unclear as no immunological correlate of protection exists. A field study conducted in a typhoid-endemic region demonstrated protection at 62% (95% CI 48%-73%) over seven years post vaccination.
Pharmacokinetic Properties
5.2 Pharmacokinetic properties Not applicable.

שימוש לפי פנקס קופ''ח כללית 1994
לא צוין
תאריך הכללה מקורי בסל
לא צוין
הגבלות
לא צוין
ATC
מידע נוסף
