Quest for the right Drug
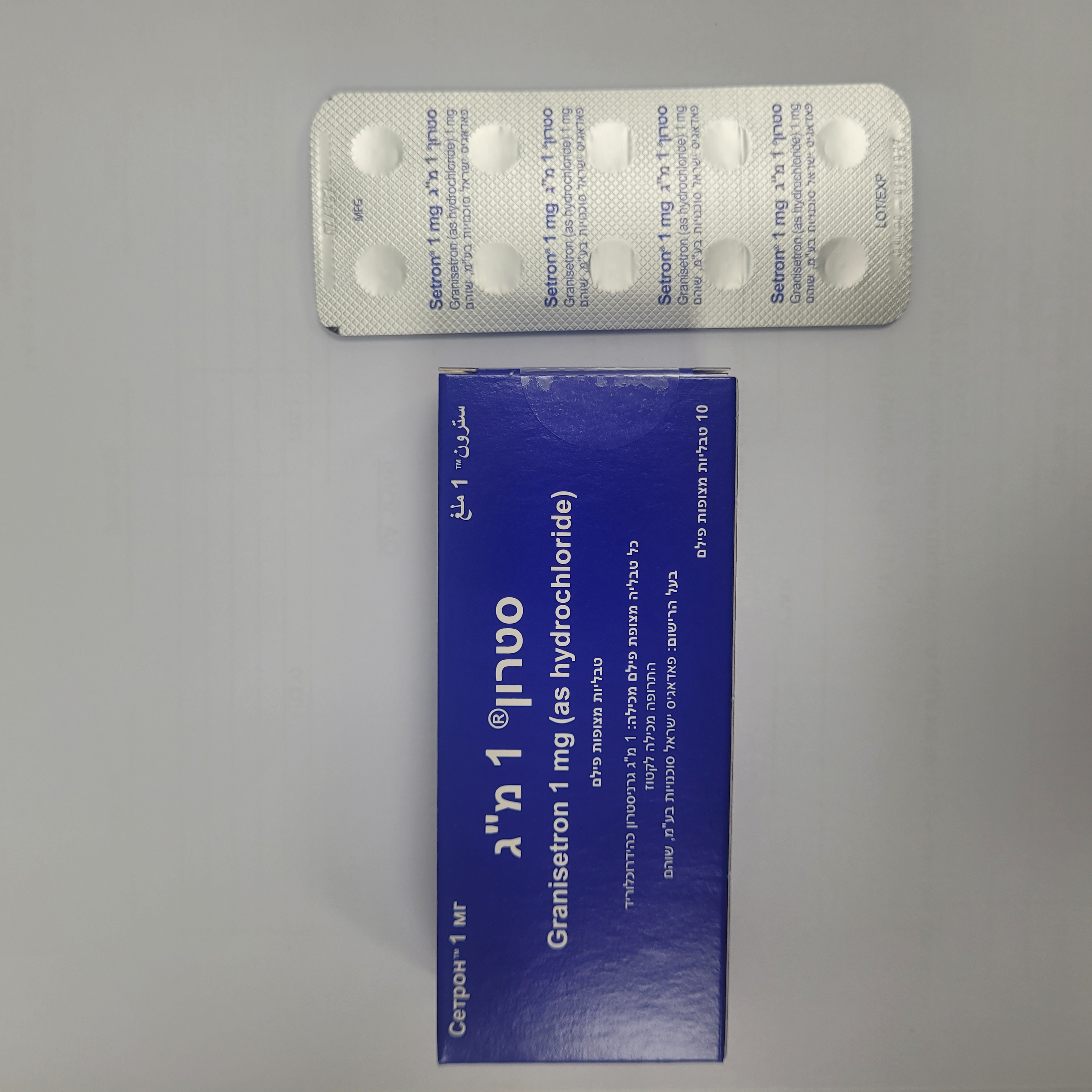
סטרון 1 מ"ג SETRON 1 MG (GRANISETRON AS HYDROCHLORIDE)
תרופה במרשם
תרופה בסל
נרקוטיקה
ציטוטוקסיקה
צורת מתן:
פומי : PER OS
צורת מינון:
טבליות מצופות פילם : FILM COATED TABLETS
עלון לרופא
מינוניםPosology התוויות
Indications תופעות לוואי
Adverse reactions התוויות נגד
Contraindications אינטראקציות
Interactions מינון יתר
Overdose הריון/הנקה
Pregnancy & Lactation אוכלוסיות מיוחדות
Special populations תכונות פרמקולוגיות
Pharmacological properties מידע רוקחי
Pharmaceutical particulars אזהרת שימוש
Special Warning עלון לרופא
Physicians Leaflet
Adverse reactions : תופעות לוואי
4.8 Undesirable effects Summary of the safety profile The most frequently reported adverse reactions for granisetron are headache and constipation, which may be transient. ECG changes including QT prolongation have been reported with granisetron (see sections 4.4 and 4.5). Tabulated list of adverse reactions The following table of listed adverse reactions is derived from clinical trials and post-marketing data associated with granisetron and other 5-HT3 antagonists. Frequency categories are as follows: Very common: (≥1/10) Common (≥1/100 to <1/10) Uncommon (≥1/1,000 to <1/100) Rare (≥1/10,000 to <1/1,000) Very rare (<1/10,000) Immune system disorders Uncommon Hypersensitivity reactions e.g. anaphylaxis, urticaria Psychiatric disorders Common Insomnia Nervous system disorders Very common Headache Uncommon Extrapyramidal Reactions Uncommon Serotonin Syndrome (see also sections 4.4 and 4.5) Cardiac disorders Uncommon QT prolongation Gastrointestinal disorders Very common Constipation Common Diarrhoea Hepatobiliary disorders Common Elevated hepatic transaminases* Skin and subcutaneous tissue disorders Uncommon Rash *Occurred at a similar frequency in patients receiving comparator therapy Description of selected adverse reactions As for other 5-HT3 antagonists, ECG changes including QT prolongation have been reported with granisetron (see sections 4.4 and 4.5). As with other 5-HT3 antagonists, cases of serotonin syndrome (including altered mental status, autonomic dysfunction and neuromuscular abnormalities) have been reported following the concomitant use of granisetron and other serotonergic drugs (see sections 4.4 and 4.5). Reporting of suspected adverse reactions Reporting suspected adverse reactions after authorisation of the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the medicinal product. Any suspected adverse events should be reported to the Ministry of Health according to the National Regulation by using an online form https://sideeffects.health.gov.il Additionally, you can also report to Padagis via the following address: Padagis.co.il.

שימוש לפי פנקס קופ''ח כללית 1994
Nausea & vomiting induced by cytostatic therapy. ירשם ע"י רופא אונקולוג לחולים אונקולוגיים בלבד
תאריך הכללה מקורי בסל
01/01/1995
הגבלות
תרופה מוגבלת לרישום ע'י רופא מומחה או הגבלה אחרת
מידע נוסף
