Quest for the right Drug
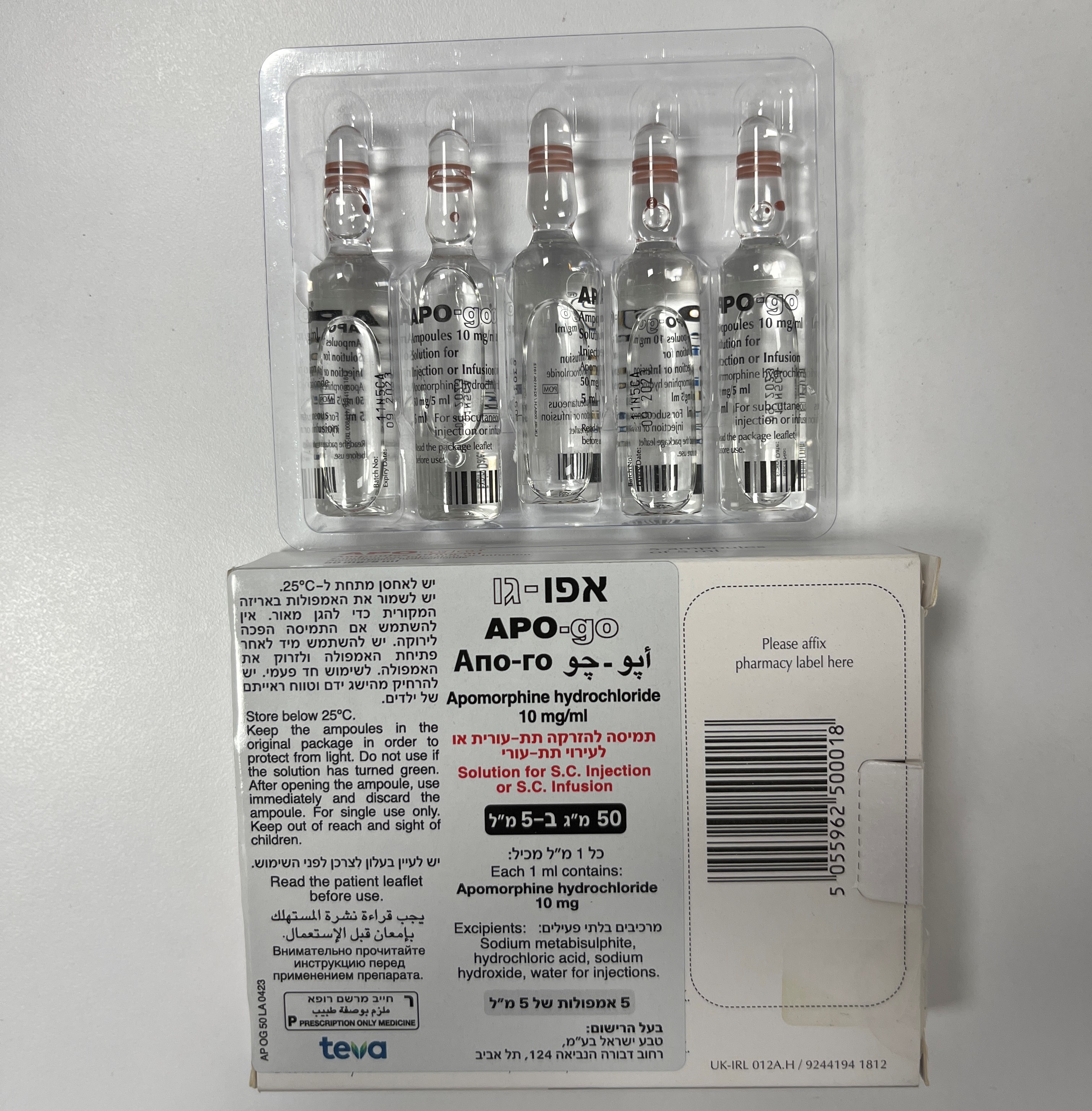
אפו-גו APO-GO (APOMORPHINE HYDROCHLORIDE)
תרופה במרשם
תרופה בסל
נרקוטיקה
ציטוטוקסיקה
צורת מתן:
תת-עורי : S.C
צורת מינון:
תמיסה להזרקהאינפוזיה : SOLUTION FOR INJECTION / INFUSION
עלון לרופא
מינוניםPosology התוויות
Indications תופעות לוואי
Adverse reactions התוויות נגד
Contraindications אינטראקציות
Interactions מינון יתר
Overdose הריון/הנקה
Pregnancy & Lactation אוכלוסיות מיוחדות
Special populations תכונות פרמקולוגיות
Pharmacological properties מידע רוקחי
Pharmaceutical particulars אזהרת שימוש
Special Warning עלון לרופא
Physicians Leaflet
Posology : מינונים
4.2. Posology and method of administration Selection of patients suitable for APO-go injections: Patients selected for treatment with APO-go should be able to recognise the onset of their ‘off’ symptoms and be capable of injecting themselves or else have a responsible carer able to inject for them when required. Patients treated with apomorphine will usually need to start domperidone at least two days prior to initiation of therapy. The domperidone dose should be titrated to the lowest effective dose and discontinued as soon as possible. Before the decision to initiate domperidone and apomorphine treatment, risk factors for QT interval prolongation in the individual patient should be carefully assessed to ensure that the benefit outweighs the risk (see Section 4.4). Refer to the domperidone prescribing information for recommended domperidone dosage information. Apomorphine should be initiated in the controlled environment of a specialist clinic. The patient should be supervised by a physician experienced in the treatment of Parkinson’s disease (e.g., neurologist). The patient’s treatment with levodopa, with or without dopamine agonists, should be optimised before starting APO-go treatment. Posology Continuous Infusion Patients who have shown a good ‘on’ period response during the initiation stage of apomorphine therapy, but whose overall control remains unsatisfactory using intermittent injections, or who require many and frequent injections (more than 10 per day), may be commenced on or transferred to continuous subcutaneous infusion by minipump and/or syringe-driver as follows: Continuous infusion is started at a rate of 1 mg apomorphine HCl (0.1 ml) per hour, then increased according to the individual response. Increases in the infusion rate should not exceed 0.5 mg per hour at intervals of not less than 4 hours. Hourly infusion rates may range between 1 mg and 4 mg (0.1 ml and 0.4 ml), equivalent to 0.015 - 0.06 mg/kg/hour. Infusions should run for waking hours only. Unless the patient is experiencing severe night-time problems, 24- hour infusions are not advised. Tolerance to the therapy does not seem to occur as long as there is an overnight period without treatment of at least 4 hours. In any event, the infusion site should be changed every 12 hours. Patients may need to supplement their continuous infusion with intermittent bolus boosts as necessary, and as directed by their physician. A reduction in dosage of other dopamine agonists may be considered during continuous infusion. Determination of the threshold dose The appropriate dose for each patient is established by incremental dosing schedules. The following schedule is suggested: 1 mg of apomorphine HCl (0.1 ml), that is approximately 15-20 micrograms/kg, may be injected subcutaneously during a hypokinetic or ‘off’ period and the patient is observed over 30 minutes for a motor response. If no response, or an inadequate response, is obtained, a second dose of 2 mg of apomorphine HCl (0.2 ml) is injected subcutaneously and the patient observed for an adequate response for a further 30 minutes. The dosage may be increased by incremental injections with at least a forty- minute interval between succeeding injections, until a satisfactory motor response is obtained. Establishment of treatment Once the appropriate dose is determined, a single subcutaneous injection may be given into the lower abdomen or outer thigh at the first signs of an ‘off’ episode. It cannot be excluded that absorption may differ with different injection sites within a single individual. Accordingly, the patient should then be observed for the next hour to assess the quality of their response to treatment. Alterations in dosage may be made according to the patient’s response. The optimal dosage of apomorphine hydrochloride varies between individuals but, once established, remains relatively constant for each patient. Precautions on continuing treatment The daily dose of APO-go varies widely between patients, typically within the range of 3-30 mg, given as 1-10 injections and sometimes as many as 12 separate injections per day. It is recommended that the total daily dose of apomorphine HCl should not exceed 100 mg and that individual bolus injections should not exceed 10 mg. In clinical studies it has usually been possible to make some reduction in the dose of levodopa; this effect varies considerably between patients and needs to be carefully managed by an experienced physician. Once treatment has been established, domperidone therapy may be gradually reduced in some patients but successfully eliminated only in a few, without any vomiting or hypotension. Paediatric population APO-go Ampoules 10 mg/ml Solution for Injection or Infusion is contraindicated for children and adolescents under 18 years of age (see Section 4.3). Elderly The elderly are well represented in the population of patients with Parkinson’s disease and constitute a high proportion of those studied in clinical trials of APO-go. The management of elderly patients treated with APO-go has not differed from that of younger patients. However, extra caution is recommended during initiation of therapy in elderly patients because of the risk of postural hypotension. Renal impairment A dose schedule similar to that recommended for adults and the elderly can be followed for patients with renal impairment (see Section 4.4). Method of administration APO-go Ampoules 10 mg/ml Solution for Injection or Infusion is for subcutaneous use by intermittent bolus injection. APO-go Ampoules 10 mg/ml Solution for Injection or Infusion may also be administered as a continuous subcutaneous infusion by minipump and/or syringe-driver (see Section 6.5). Apomorphine must not be used via the intravenous route. Do not use if the solution has turned green. The solution should be inspected visually prior to use. Only clear, colourless and particle-free solution should be used.

פרטי מסגרת הכללה בסל
התרופה תינתן לטיפול במחלת פרקינסון אשר איננה נשלטת על אף התאמה אישית של מינון עם LEVODOPA או אגוניסטים דופמינרגיים אחרים
מסגרת הכללה בסל
התוויות הכלולות במסגרת הסל
| התוויה | תאריך הכללה | תחום קליני | Class Effect | מצב מחלה |
|---|---|---|---|---|
| התרופה תינתן לטיפול במחלת פרקינסון אשר איננה נשלטת על אף התאמה אישית של מינון עם LEVODOPA או אגוניסטים דופמינרגיים אחרים | 01/03/2008 |
שימוש לפי פנקס קופ''ח כללית 1994
לא צוין
תאריך הכללה מקורי בסל
01/03/2008
הגבלות
תרופה מוגבלת לרישום ע'י רופא מומחה או הגבלה אחרת
מידע נוסף
עלון מידע לצרכן
06.10.21 - עלון לצרכן אנגלית 06.10.21 - עלון לצרכן עברית 06.10.21 - עלון לצרכן ערבית 06.03.24 - עלון לצרכן עברית 18.06.24 - עלון לצרכן אנגלית 18.06.24 - עלון לצרכן עברית 18.06.24 - עלון לצרכן ערבית 12.06.13 - החמרה לעלון 29.07.18 - החמרה לעלון 18.04.21 - החמרה לעלון 06.03.24 - החמרה לעלוןלתרופה במאגר משרד הבריאות
אפו-גו