Quest for the right Drug
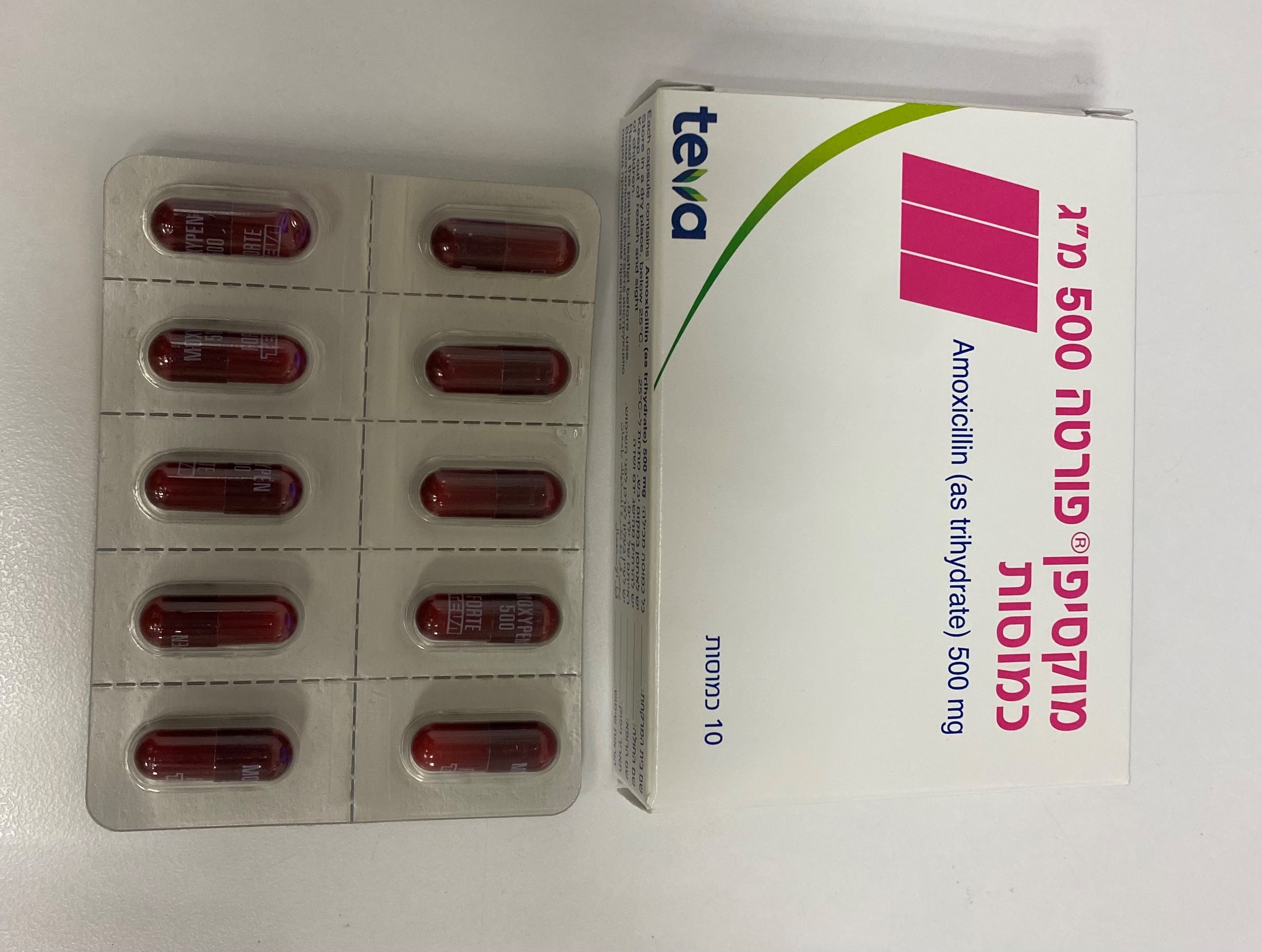
מוקסיפן ® פורטה 500 מ"ג כמוסות MOXYPEN ® FORTE 500 MG CAPSULES (AMOXICILLIN AS TRIHYDRATE, AMOXICILLIN TRIHYDRATE)
תרופה במרשם
תרופה בסל
נרקוטיקה
ציטוטוקסיקה
צורת מתן:
פומי : PER OS
צורת מינון:
קפסולות : CAPSULES
עלון לרופא
מינוניםPosology התוויות
Indications תופעות לוואי
Adverse reactions התוויות נגד
Contraindications אינטראקציות
Interactions מינון יתר
Overdose הריון/הנקה
Pregnancy & Lactation אוכלוסיות מיוחדות
Special populations תכונות פרמקולוגיות
Pharmacological properties מידע רוקחי
Pharmaceutical particulars אזהרת שימוש
Special Warning עלון לרופא
Physicians Leaflet
Posology : מינונים
4 DOSAGE AND ADMINISTRATION With the exception of gonorrhea, treatment with Moxypen should be continued for a minimum of 48‐72 hours beyond the time at which the patient becomes asymptomatic or evidence of bacterial eradication has been obtained. In the treatment of group A β‐hemolytic streptococcal infections, therapy with this drug should be continued for at least 10 days to help prevent the occurrence of acute rheumatic fever or glomerulonephritis. Upper Respiratory Tract and Chest Infections Adults and Children over 10 Years of Age: The recommended dosage is 250‐500 mg 3 times daily, every 8 hours. Infants and Children under 10 Years of Age: In infants under 2 years of age, the dosage is 62.5 mg 3 times daily, every 8 hours. In children 2‐10 years of age, the dosage is 125 mg 3 times daily, every 8 hours. For more severe infections, the dosage may be increased to 250 mg 3 times daily. The recommended dosage according to body weight is 20 mg/kg per day in divided doses every 8 hours. For more severe infections, the dosage may be increased to 40 mg/kg body weight per day every 8 hours. Skin and Soft‐tissue Infections Treat as for upper respiratory tract and chest infections. Uncomplicated Lower Urinary Tract Infections Adults A single dose of 3 g may be administered. Children A single dose of 100 mg/kg body weight may be administered. Gonorrhea A single dose of 3 g may be administered. Prophylaxis of Bacterial Endocarditis (in dental procedures) Adults and Children over 10 Years of Age A single dose of 3 g about 1 hour prior to the procedure, to prevent bacteremia. Children under 10 Years of Age Half the adult dose. In order to obtain optimal absorption of drug from Moxypen Forte capsules they should be administered between meals with a glass of water (250 mL or 8 fl. oz.). 4.1 Reconstitution Directions for Dispensing Oral Suspension: Any unused medicinal product or waste material should be disposed of in accordance with local requirements. Prepare these formulations at the time of dispensing. For ease in preparation, add water to the bottle in two portions and shake well after each addition. Add the total amount of water as directed on the labeling of the package being dispensed: 36 ml or 60 ml of distilled water to prepare 60 ml or 100 ml of suspension accordingly. The reconstituted formulation is stable for 14 days under refrigeration (2‐8°C) or 14 days at room temperature (25°C). 4.2 Administration In order to obtain optimal absorption of drug from Moxypen Forte capsules they should be administered between meals with a glass of water (250 mL or 8 fl. oz.). 4.3 Missed Dose Patients should be instructed to take Moxypen Forte at the next scheduled dose and not take two doses at the same time if they miss a dose. 5 OVERDOSAGE Treatment of overdosage would likely be needed only in patients with severely impaired renal function since patients with normal kidneys excrete penicillins at a fast rate. Hemodialysis would, therefore, represent the main form of treatment. Activated charcoal may be administered to aid in the removal of unabsorbed drug. General supportive measures are recommended. For management of a suspected drug overdose, immediately contact your doctor. 6 DOSAGE FORMS, STRENGTHS, COMPOSITION AND PACKAGING Table‐1 ‐ Dosage Forms, Strengths, Composition and Packaging Dosage Form / Strength Inactive ingredients Route of Administration / Composition Oral 500 mg Capsules Magnesium stearate Capsule shell: gelatin, FD&C red #3 (erythrosine E127), titanium dioxide, FD&C blue # 2 (E132). Printing ink: titanium dioxide, dehydrated alcohol, isopropyl alcohol, shellac, povidone, butyl alcohol, propylene glycol, sodium hydroxide. Oral Powder for Suspension Sucrose, spray dried artificial flavor 250 mg/5ml (cherry raspberry type), silicon dioxide, sodium citrate anhydrous, xanthan gum, sodium benzoate, FD&C red # 40. Moxypen Forte 500mg Capsules: 500 mg amoxicillin (as the trihydrate) in hard gelatin capsule with Opaque maroon cap and opaque maroon body. Printed white MOXYPEN over 500 on the cap and FORTE over Teva logo on body portion of the capsule. Packed in bottle of 20 capsules or in blisters of 10 or 20 capsules. Not all pack sizes/type may be marketed. Moxypen Forte 250 mg Powder for Suspension: Finely granulated, off white to pinkish powder. After adding water, a pink‐colored liquid will be obtained. Packed in two package sizes: 60 ml or 100 ml of suspension. Not all pack sizes may be marketed. 7 WARNINGS AND PRECAUTIONS General If superinfections with mycotic or bacterial pathogens occur (usually involving Aerobacter, Pseudomonas or Candida) treatment with Moxypen Forte should be discontinued and appropriate therapy instituted. Moxypen Forte is contraindicated in cases where infectious mononucleosis is either suspected or confirmed (see 2 CONTRAINDICATIONS). A morbilliform rash following the use of ampicillin in patients with infectious mononucleosis has been well documented and has also been reported to occur following the use of amoxicillin. Cardiovascular Kounis syndrome, a serious allergic reaction that can result in myocardial infarction, can occur as chest pain in association with an allergic reaction to amoxicillin. Gastrointestinal Clostridium difficile‐associated disease Clostridium difficile‐associated disease (CDAD) has been reported with use of many antibacterial agents, including amoxicillin (see 8 ADVERSE REACTIONS). CDAD may range in severity from mild diarrhea to fatal colitis. It is important to consider this diagnosis in patients who present with diarrhea, or symptoms of colitis, pseudomembranous colitis, toxic megacolon, or perforation of colon subsequent to the administration of any antibacterial agent. CDAD has been reported to occur over 2 months after the administration of antibacterial agents. Treatment with antibacterial agents may alter the normal flora of the colon and may permit overgrowth of Clostridium difficile. C. difficile produces toxins A and B, which contribute to the development of CDAD. CDAD may cause significant morbidity and mortality. CDAD can be refractory to antimicrobial therapy. If the diagnosis of CDAD is suspected or confirmed, appropriate therapeutic measures should be initiated. Mild cases of CDAD usually respond to discontinuation of antibacterial agents not directed against Clostridium difficile. In moderate to severe cases, consideration should be given to management with fluids and electrolytes, protein supplementation, and treatment with an antibacterial agent clinically effective against Clostridium difficile. Surgical evaluation should be instituted as clinically indicated, as surgical intervention may be required in certain severe cases. Hematologic Periodic assessment of hematopoietic function should be made during prolonged therapy with Moxypen Forte (amoxicillin). Hepatic/Biliary/Pancreatic A moderate rise in serum glutamic oxaloacetic transaminase (SGOT) has been noted. Periodic assessment of hepatic function should be made during prolonged therapy with Moxypen Forte (amoxicillin). (See 7.1.2 Pediatrics; 8.2 Clinical Trial Adverse Reactions) Susceptibility/Resistance Development of Drug Resistant Bacteria Prescribing Moxypen Forte in the absence of a proven or strongly suspected bacterial infection is unlikely to provide benefit to the patient and risks the development of drug‐resistant bacteria. Immune Hypersensitivity Serious and occasionally fatal hypersensitivity (anaphylactoid) reactions have been reported in patients on penicillin therapy. Although anaphylaxis is more frequent following parenteral therapy, it has occurred in patients following oral dosing of penicillins. These reactions are more likely to occur in patients with a history of hypersensitivity to beta‐lactams and individuals with a history of sensitivity to multiple allergens. There have been well‐documented reports of individuals with a history of penicillin hypersensitivity reactions who have experienced severe hypersensitivity reactions when treated with cephalosporins. Before initiating therapy with a penicillin, careful inquiry should be made concerning previous hypersensitivity reactions to penicillins, cephalosporins and other allergens. If an allergic reaction occurs, administration of Moxypen Forte (amoxicillin) should be discontinued and appropriate therapy instituted. Serious anaphylactoid reactions require immediate emergency treatment with epinephrine. Oxygen, intravenous steroids, and airway management, including intubation, should also be administered as indicated. Monitoring and Laboratory Tests Periodic assessment of renal, hepatic and hematopoietic functions should be made during prolonged therapy with Moxypen Forte (amoxicillin). Abnormal prolongation of prothrombin time (increased international normalized ratio (INR)) has been reported in patients receiving amoxicillin and oral anticoagulants. Appropriate monitoring should be undertaken when amoxicillin and oral anticoagulants are prescribed concurrently, particularly upon initiation or cessation of concurrent administration. Adjustments in the dose of oral anticoagulants may be necessary to maintain the desired level of anticoagulation. Renal Because amoxicillin is excreted mostly by the kidney, the dosage for patients with renal impairment should be reduced in proportion to the degree of loss of renal function Periodic assessment of renal function should be made during prolonged therapy with Moxypen Forte (amoxicillin). Skin Severe Cutaneous Adverse Reactions Severe cutaneous adverse reactions (SCAR) such as acute generalized exanthematous pustulosis (AGEP), drug reaction with eosinophilia and systemic symptoms (DRESS), Stevens‐Johnson syndrome (SJS), and toxic epidermal necrolysis (TEN) have been reported in association with beta‐lactam treatment. When SCAR is suspected, Moxypen Forte should be discontinued and appropriate therapy and/or measures should be taken. 7.1 Special Populations 7.1.1 Pregnant Women The safety of Moxypen Forte in the treatment of infections during pregnancy has not been established. If the administration of Moxypen Forte to pregnant patients is considered to be necessary, its use requires that the potential benefits be weighed against the possible hazards to the fetus. 7.1.2 Pediatrics Pediatrics (<18): A moderate rise in serum glutamic oxaloacetic transaminase (SGOT) has been noted, particularly in infants, but the significance of this finding is not known. 7.1.3 Geriatrics Use in the Elderly: There are no known specific precautions for the use of amoxicillin in the elderly. Amoxicillin is known to be substantially excreted by the kidney, and the risk of toxic reactions to this Moxypen Forte may be greater in patients with impaired renal function. Because elderly patients are more likely to have decreased renal function, care should be taken in dose selection and it may be useful to monitor renal function. 7.2 Important information about some of the excipients‐ Moxypen Forte 250 Suspension: Moxypen Forte 250 Suspension contains about 3 g sucrose per 5 ml. Therefore, patients with rare hereditary problems of fructose intolerance, glucose‐galactose malabsorption or sucrase‐ isomaltase insufficiency should not take this medicine. Moxypen Forte 250 mg Powder for Suspension contains 2.79 mg sodium benzoate (E211) in each 5 ml. Sodium benzoate may increase jaundice (yellowing of the skin and eyes) in newborn babies (up to 4 weeks old). Moxypen Forte 250 mg Powder for Suspension contains about 1.5mg benzyl alcohol, which may cause allergic reactions. Benzyl alcohol has been linked with the risk of severe side effects including breathing problems (called “gasping syndrome”) in young children. Do not give to newborn baby (up to 4 weeks old), unless recommended by the doctor. Do not use for more than a week in young children (less than 3 years old), unless advised by the doctor. Ask your doctor or pharmacist for advice if you have a liver or kidney disease. This is because large amounts of benzyl alcohol can build‐up in your body and may cause side effects (called “metabolic acidosis”). Ask your doctor or pharmacist for advice if you are pregnant or breast-feeding. This is because large amounts of benzyl alcohol can build‐up in your body and may cause side effects (called “metabolic acidosis”). Moxypen Forte 250 Suspension contains FD&C red #40 (E129) which may cause allergic reactions. Moxypen Forte 250 mg Powder for Suspension contains 3.20 mg per 5 ml, less than 1 mmol sodium (23 mg) per 5 ml, that is to say essentially "sodium‐free". 8 ADVERSE REACTIONS 8.1 Adverse Reaction Overview As with other penicillins, it may be expected that untoward reactions will be related to sensitivity phenomena. They are more likely to occur in individuals who have previously demonstrated hypersensitivity to penicillins and cephalosporins and in those with a history of allergy, asthma, hay fever or urticaria. 8.2 Clinical Trial Adverse Reactions The following adverse reactions have been reported as associated with the use of Moxypen Forte: Gastrointestinal ‐ Nausea, vomiting and diarrhea, hemorrhagic and pseudomembranous colitis. Clostridium difficile‐associated disease (CDAD) has been reported with use of many antibacterial agents, including amoxicillin. Glossitis, black "hairy" tongue and stomatitis, mucocutaneous candidiasis, tooth discoloration (brown, yellow or gray staining); most reports occurred in pediatric patients. Discoloration was reduced or eliminated with brushing or dental cleaning in most cases. Hypersensitivity Reactions ‐ Skin rashes have been reported frequently. Less commonly, a few cases of serum sickness like reactions including urticaria, erythema, erythema multiforme, angioneurotic edema, pruritus have been reported. Rarely, Stevens‐Johnson syndrome, toxic epidermal necrolysis, bullous dermatitis, exfoliative dermatitis, acute generalized exanthematous pustulosis, hypersensitivity vasculitis have been reported. Anaphylaxis is the most serious reaction experienced and has usually been associated with the parenteral dosage form. NOTE: Urticaria, other skin rashes, and serum sickness‐like reactions may be controlled with antihistamines and if necessary, systemic corticosteroids. Whenever such reactions occur, Moxypen Forte (amoxicillin) should be discontinued unless, in the opinion of the physician, the condition being treated is life threatening and amenable only to amoxicillin therapy. Serious anaphylactic reactions require the immediate use of epinephrine, oxygen and intravenous steroids. Hepatobiliary ‐ A moderate rise in serum glutamic oxaloacetic transaminase (SGOT) has been noted, particularly in infants, but the significance of this finding is not known. Transient increases in serum alkaline phosphatase and lactic dehydrogenase levels have also been observed but they returned to normal on discontinuation of amoxicillin. Reports have also been seen of hepatic dysfunction including cholestatic jaundice, hepatic cholestasis, acute cytolytic hepatitis, Hemic and Lymphatic Systems ‐ Anemia thrombocytopenia, thrombocytopenic purpura, eosinophilia, leukopenia, neutropenia and agranulocytosis have been reported during therapy with the penicillins. These reactions are usually reversible on discontinuation of therapy and are believed to be a hypersensitivity phenomena. Reports have also been seen of anemia including hemolytic anemia. Central Nervous System ‐ As with other penicillins, acute and chronic toxicity is not a clinical problem. Although penicillins do not normally cross the blood‐brain barrier to any substantial extent, if massive doses are given (several grams per day) to elderly patients, patients with inflamed meninges or patients with impaired renal function, toxic reactions are likely to occur. At extremely high doses, convulsions can occur. When penicillin reaches a high concentration in the cerebrospinal fluid, neurotoxic symptoms consisting of myoclonia, convulsive seizures and depressed consciousness may occur. Unless administration of the drug is stopped or its dosage reduced, the syndrome may progress to coma and death. Dizziness, hyperkinesias, hyperactivity, agitation, anxiety, insomnia, confusion, and behavioural changes have also been reported. Skin and Appendages ‐ erythematous maculopapular rash. Renal ‐ Crystalluria. Interstitial nephritis (oliguria, proteinuria, hematuria, hyaline casts, pyuria) and nephropathy are infrequent and usually associated with high doses of parenteral penicillins; however, this has occurred with all of the penicillins. Such reactions are hypersensitivity responses and are usually associated with fever, skin rash and eosinophilia. Elevations of creatinine or blood urea nitrogen may occur. 8.3 Post‐Marketing Adverse Reactions Central Nervous System: Amoxicillin can lead to cases of aseptic meningitis of unknown frequency. Other immune system disorders: Kounis syndrome 8.4 Reporting of suspected adverse reactions Reporting suspected adverse reactions after authorisation of the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the medicinal product. Any suspected adverse events should be reported to the Ministry of Health according to the National Regulation by using an online form: https://sideeffects.health.gov.il 9 DRUG INTERACTIONS 9.1 Drug‐Drug Interactions Methotrexate: Penicillins compete with renal tubular secretion of methotrexate, resulting in decreased clearance of methotrexate. Concomitant use may increase methotrexate serum concentrations, with increased risk of toxicity. Probenecid: Probenecid inhibits the renal tubular excretion of amoxicillin. Concurrent use of amoxicillin and probenecid may result in increased and prolonged blood levels of amoxicillin. Warfarin: Abnormal prolongation of prothrombin time (increased international normalized ratio [INR]) has been reported in patients receiving amoxicillin and warfarin. Appropriate monitoring should be undertaken when warfarin is prescribed concurrently. Adjustments in the dose of oral anticoagulants may be necessary to maintain the desired level of anticoagulation. Oral Contraceptives: Moxypen Forte may affect the gut flora, leading to lower estrogen reabsorption and reduced efficacy of combined oral estrogen/progesterone contraceptives. Tetracyclines: Bacteriostatic action of tetracyclines may inhibit bactericidal activity of penicillins. 9.2 Drug‐Food Interactions Amoxicillin is stable in the presence of gastric acid. Moxypen Forte is rapidly and well absorbed after oral administration to fasting subjects. It was found in a recent study that peak serum antibiotic levels were reduced by 50% in subjects receiving amoxicillin immediately following a standard meal. Reducing the dose‐water volume given with amoxicillin from 250 to 25 mL in fasted subjects also caused a significant reduction in serum amoxicillin levels. This may be due to the low water solubility of amoxicillin trihydrate (1 g in 370 mL water). In addition, food ingestion immediately before dosing also reduced the urinary excretion. 9.3 Drug‐Herb Interactions Interactions with herbal products have not been established. 9.4 Drug‐Laboratory Test Interactions Moxypen Forte may: • cause false‐positive reactions when testing for the presence of glucose in urine. • distort assay results for estriol in pregnant women. 10 ACTION AND CLINICAL PHARMACOLOGY 10.1 Mechanism of Action Moxypen Forte (amoxicillin) exerts its bactericidal action by interfering with bacterial cell wall synthesis. 10.2 Pharmacokinetics Peak serum levels are attained between 1 and 2 hours after drug administration. Amoxicillin diffuses readily into most body tissues and fluids, with the exception of brain and spinal fluid. Amoxicillin is excreted largely unchanged in the urine while 10‐25% of the administered dose is excreted in the form of penicilloic acid. The excretion of amoxicillin can be delayed by concurrent administration of probenecid. Amoxicillin is not highly protein bound. In blood serum, amoxicillin is approximately 17‐1 8% protein bound compared to 59% for penicillin G. The following amoxicillin mean serum levels were found following the administration of 250 mg capsules of Moxypen Forte to 12 healthy adult volunteers: Time (hr.) 0.6 1.0 1.5 2 3 4 5 7 Mean Serum Levels 0.81 2.96 3.17 3.10 2.22 1.12 0.50 0.11 Peak blood serum levels averaged 3.8µg/mL (range 2.35 to 6.38) and the Tmax was 1.50 hr. The mean biological half‐life (t %) was found to be 55.8 minutes with a mean elimination rate constant Kel of 0.7456 hr.‐1. Twelve normal male subjects participated in a bioavailability study of Moxypen Forte powder for Suspension. Each subject was given 5 mL (250 mg) of reconstituted Moxypen Forte powder for Suspension in a single dose. The following amoxicillin mean serum levels were found: Time (hr.) 0.5 1.0 1.5 2 3 4 5 7 3.26 4.19 3.40 2.56 1.65 0.98 0.43 0.10 Mean Serum Levels (µg/mL) Peak plasma concentrations from 2.65 to 5.75 μg /mL were obtained with a mean Cmax of 4.24 ± 0.74 μg /mL. The time required to reach peak concentrations ranged from 0.5 to 1.5 hours, with a Tmax mean of 1 .00 ± 0.21 hr. The AUC's calculated for 0 to 7 hours ranged from 8.475 to 12.865 μg‐hours/mL. The mean AUC was 10.71 3 ± 1.443 μg‐hours/mL. The mean biological half‐life for Moxypen Forte powder for Suspension was 26.4 minutes. The mean elimination rate constant (Kel) was 1.57 hour‐1. The administration of 500 mg amoxicillin to healthy fasting subjects has been reported to produce peak mean serum levels of 10.8 μg/mL and 6.75 μg/mL. Additional studies in healthy volunteers with normal renal function receiving 500 mg doses, indicated that peak serum levels could vary from 5.0 to 10.8 μg/mL. Serum amoxicillin half‐life values reported in the literature vary from 1‐1.3 hours. About 60‐80% of an oral dose of amoxicillin is excreted in the urine. In the presence of renal impairment, the serum half‐life increases (between 7 and 10 hours), necessitating a reduction in the dosage administered. 11 STORAGE, STABILITY AND DISPOSAL Shelf life of unopened packages: The expiry date of the product is indicated on the packaging materials. Moxypen Forte 500 mg Capsules: Store in a dry place below 25°C. Shelf life after opening (bottle presentation): to be used within 1 month after first opening the bottle. Moxypen Forte 250 mg Powder for Suspension: Store the powder in a dry place, below 25°C. Shelf life after reconstitution: The reconstituted suspension should be kept in the refrigerator (2°‐8°C) or at room temperature (25°C) and should be used within 14 days.

שימוש לפי פנקס קופ''ח כללית 1994
respiratory infections (otitis, sinusitis, bronchitis & pneumonia) gastrointestinal infections, genitourinary tract infections, gonorrhea, soft tissue infections caused by: streptococci, pneumococci, staphylococci, enterococci, H. influenzae, E. coli, proteus, gonococci, shigella, salmonella. prophylaxis of endocarditis.
תאריך הכללה מקורי בסל
01/01/1995
הגבלות
תרופה שאושרה לשימוש כללי בקופ'ח
מידע נוסף
עלון מידע לצרכן
04.01.22 - עלון לצרכן אנגלית 04.01.22 - עלון לצרכן עברית 04.01.22 - עלון לצרכן ערבית 21.08.23 - עלון לצרכן עברית 28.12.23 - עלון לצרכן עברית 28.01.24 - עלון לצרכן אנגלית 28.01.24 - עלון לצרכן עברית 28.01.24 - עלון לצרכן ערבית 09.03.14 - החמרה לעלון 06.10.21 - החמרה לעלון 18.10.21 - החמרה לעלון 03.01.22 - החמרה לעלון 21.08.23 - החמרה לעלוןלתרופה במאגר משרד הבריאות
מוקסיפן ® פורטה 500 מ"ג כמוסות