Quest for the right Drug
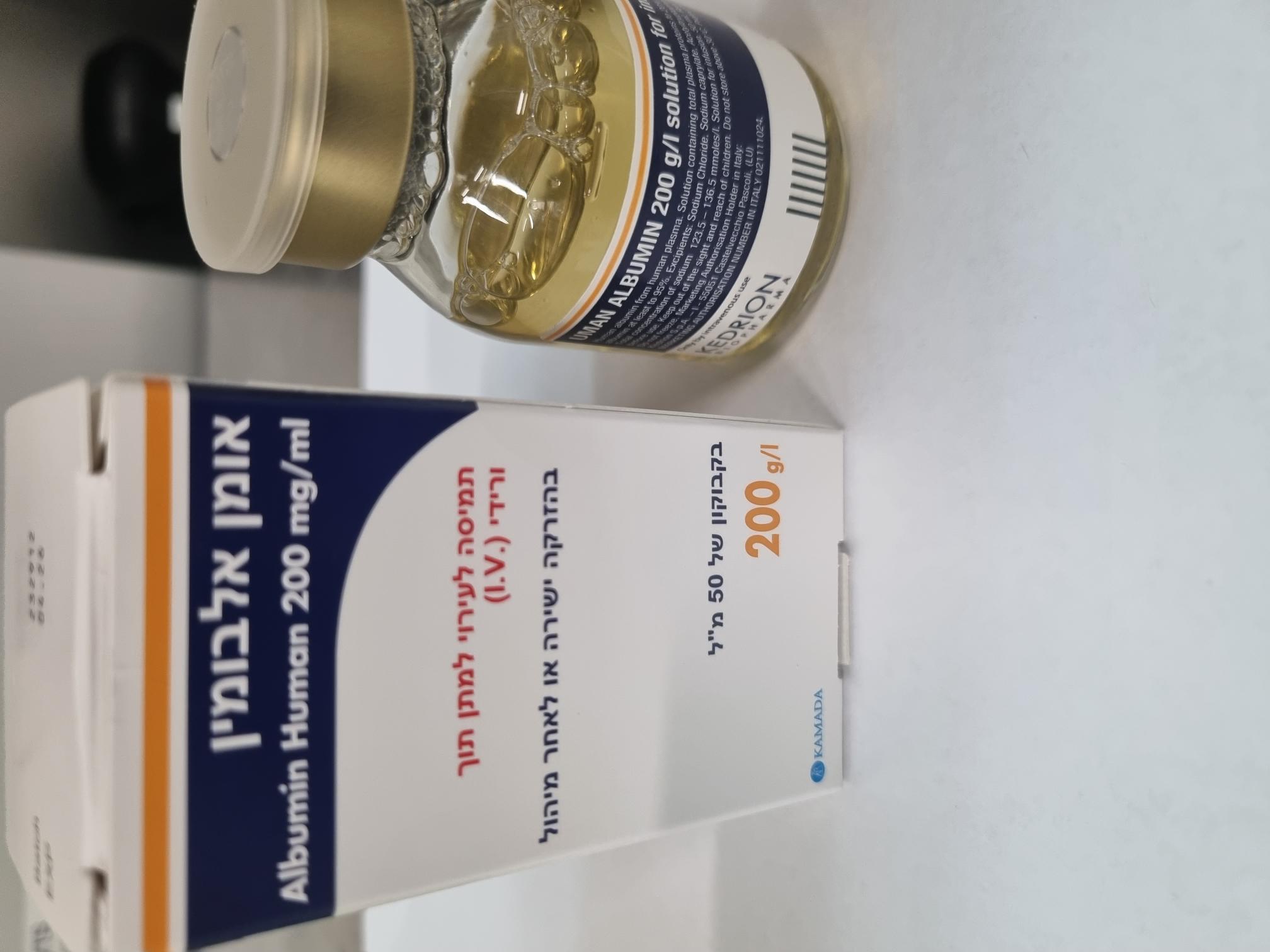
אומן אלבומין UMAN ALBUMIN (ALBUMIN HUMAN)
תרופה במרשם
תרופה בסל
נרקוטיקה
ציטוטוקסיקה
צורת מתן:
תוך-ורידי : I.V
צורת מינון:
תמיסה לאינפוזיה : SOLUTION FOR INFUSION
עלון לרופא
מינוניםPosology התוויות
Indications תופעות לוואי
Adverse reactions התוויות נגד
Contraindications אינטראקציות
Interactions מינון יתר
Overdose הריון/הנקה
Pregnancy & Lactation אוכלוסיות מיוחדות
Special populations תכונות פרמקולוגיות
Pharmacological properties מידע רוקחי
Pharmaceutical particulars אזהרת שימוש
Special Warning עלון לרופא
Physicians Leaflet
Adverse reactions : תופעות לוואי
4.8 Undesirable effects Summary of safety profile Mild reactions such as flush, urticaria, fever, and nausea occur rarely. These reactions normally disappear rapidly when the infusion rate is slowed down or the infusion is stopped. Very rarely, severe reactions, such as shock, may occur. In these cases, the infusion should be stopped and an appropriate treatment should be initiated. Tabulated list of adverse reactions The table presented below is according to the MedDRA System Organ Classification (SOC) and Preferred Term Level (PT) and it includes undesirable effects occurring with the use of human albumin solutions. Frequencies have been evaluated according to the following convention: very common (≥1/10); common (≥1/100 to <1/10); uncommon (≥1/1,000 to <1/100); rare (≥1/10,000 to <1/1,000); very rare (<1/10,000), not known (cannot be estimated from the available data). There are no robust data on the frequency of undesirable effects from clinical trials. The following data is in line with the safety profile of human albumin solutions, and confirmed by the post marketing experience; as the post marketing reporting of adverse reactions is voluntary and from a population of uncertain size, it is not possible to reliably estimate the frequency of these reactions: MedDRA System Organ Adverse reactions Frequency Class (SOC) (MedDRA Preferred Term) Hypotension Not known Vascular disorders Nervous system disorder Tremor Not known Respiratory, thoracic and Dyspnea Not known mediastinal disorder Erythema Not known Skin and subcutaneous tissue disorder Urticaria Not known Pruritus Not known Chills Not known General disorders and administration site conditions Pyrexia Not known For safety information with respect to transmissible agents, see section 4.4. Paediatric population No specific data are available on paediatric population. Reporting of suspected adverse reactions Reporting suspected adverse reactions after authorisation of the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the medicinal product. Any suspected adverse events should be reported to the Ministry of Health according to the National Regulation by using an online form: https://sideeffects.health.gov.il Additionally, you should also report to Kamada Ltd. to email address: pharmacovigilance@kamada.com

שימוש לפי פנקס קופ''ח כללית 1994
לא צוין
תאריך הכללה מקורי בסל
01/01/1995
הגבלות
תרופה מוגבלת לשימוש בבתי חולים או אשפוז יום
מידע נוסף
