Quest for the right Drug
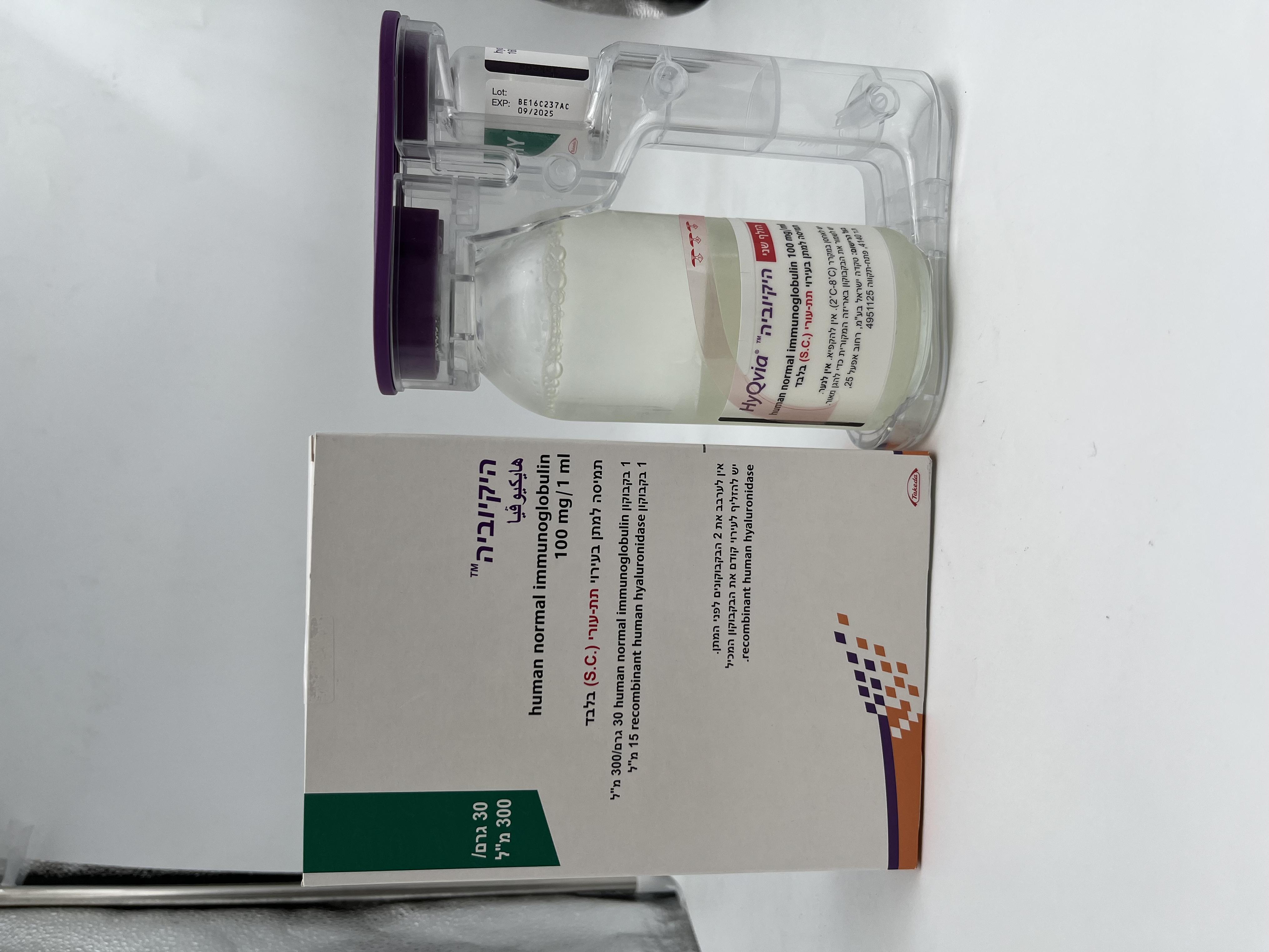
היקיוביה HYQVIA (HUMAN NORMAL IMMUNOGLOBULIN)
תרופה במרשם
תרופה בסל
נרקוטיקה
ציטוטוקסיקה
צורת מתן:
תת-עורי : S.C
צורת מינון:
תמיסה לאינפוזיה : SOLUTION FOR INFUSION
עלון לרופא
מינוניםPosology התוויות
Indications תופעות לוואי
Adverse reactions התוויות נגד
Contraindications אינטראקציות
Interactions מינון יתר
Overdose הריון/הנקה
Pregnancy & Lactation אוכלוסיות מיוחדות
Special populations תכונות פרמקולוגיות
Pharmacological properties מידע רוקחי
Pharmaceutical particulars אזהרת שימוש
Special Warning עלון לרופא
Physicians Leaflet
Adverse reactions : תופעות לוואי
4.8 Undesirable effects Summary of the safety profile The most frequently reported adverse reactions (Ars) of HyQvia were local reactions. The most frequently reported systemic Ars were headache, fatigue, and pyrexia. The majority of these Ars were mild to moderate. IG 10% Adverse reactions such as chills, headache, dizziness, fever, vomiting, allergic reactions, nausea, arthralgia, low blood pressure and moderate low back pain may occur occasionally. Rarely human normal immunoglobulins may cause a sudden fall in blood pressure and, in isolated cases, anaphylactic shock, even when the patient has shown no hypersensitivity to previous administration. Local reactions at infusion sites: swelling, soreness, redness, induration, local heat, itching, bruising and rash, may frequently occur. Cases of transient aseptic meningitis, transient haemolytic reactions, increase in serum creatinine level and/or acute renal failure have been observed with human normal immunoglobulin, see section 4.4. Thromboembolic reactions such as myocardial infarction, stroke, pulmonary embolism, and deep vein thrombosis have been rarely observed with IVIg and SCIg products. rHuPH20 The most frequent adverse reactions reported during post-marketing use of rHuPH20 in similar formulations administered subcutaneously for the dispersion and absorption of subcutaneously administered fluids or medicinal products have been mild local infusion site reactions such as erythema and pain. Oedema has been reported most frequently in association with large volume subcutaneous fluid administration. Antibodies against rHuPH20 A total of 13 out of 83 subjects who participated in pivotal PID study developed an antibody capable of binding to rHuPH20 at least once during the clinical study. These antibodies were not capable of neutralizing rHuPH20. No temporal association between adverse reactions and the presence of anti- rHuPH20 antibodies could be demonstrated. There was no increase in incidence or severity of adverse reactions in patients who developed antibodies to rHuPH20. Tabulated list of adverse reactions The safety of HyQvia was evaluated in 4 clinical studies (160602, 160603, 160902, and 161101) in 124 unique patients with PID receiving 3,202 infusions. The table presented below is according to the MedDRA System Organ Classification (SOC and Preferred Term Level). Frequencies per infusion have been evaluated using the following convention: Very common (≥ 1/10), common (≥ 1/100 to < 1/10), uncommon (≥ 1/1 000 to < 1/100), rare (≥ 1/10 000 to < 1/1 000), very rare (< 1/10 000), not known (cannot be estimated from available data). Within each frequency grouping, undesirable effects are presented in order of decreasing seriousness. Frequency of Adverse Reactions (ADRs) with HyQvia MedDRA Very common Common Uncommon Rare System Organ Class (SOC) Gastrointestinal Vomiting, nausea, disorders abdominal pain Abdominal distension (including abdominal upper and lower pain and tenderness), diarrhoea General Local reactions Local reactions (total): Local reactions Burning disorders and (total) a: Infusion site erythema, (total): sensation administration Infusion site pain infusion site swelling Infusion site site conditions (including (including local swelling discoloration, infusion discomfort, and oedema), infusion site bruising tenderness, groin site pruritus (including (including hematoma, pain) vulvovaginal pruritus) haemorrhage), infusion site mass Pyrexia, asthenic (including nodule), conditions (including infusion site warmth, asthenia, fatigue, infusion site lethargy, malaise) induration, gravitational oedema/genital swellingb (including genital oedema, scrotal and, vulvovaginal swelling) Oedema (including peripheral, swelling), chills, hyperhidrosis Investigations Direct Coombs’ test positive Musculoskeletal Myalgia, Arthralgia, back pain, and connective musculoskeletal chest pain in extremity tissue disorders pain Nervous system Headache Migraine Paresthesia disorders dizziness Skin and Erythema, rash subcutaneous (including tissue disorders erythematous, papular, maculo-papular), pruritus, urticaria Vascular Hypertension, blood disorders pressure increase Renal and Hemosiderinuria urinary disorders a The following ADRs are not listed but also calculated in the frequency for Local reactions: feeling hot, infusion site paresthesia. b Gravitational oedema/genital swellin was observed subsequent to lower abdominal quadrants administration. In addition to the adverse reactions noted in clinical trials, the following adverse reactions have been reported in the post-marketing experience (frequency of these reactions is not known (cannot be estimated from the available data)): Infections and infestations: Meningitis aseptic Immune system disorders: Hypersensitivity General disorders and administration site conditions: Influenza-like illness, infusion site leakage In addition to the adverse reactions listed above, the following additional adverse reactions have been reported for subcutaneously administered immunoglobulin products: Anaphylactic shock, anaphylactic/anaphylactoid reaction, tremor, tachycardia, hypotension, flushing, pallor, peripheral coldness, dyspnoea , paraesthesia oral, swelling face, dermatitis allergic, musculoskeletal stiffness, injection site urticaria, injection site rash, alanine aminotransferase increased. Description of selected adverse reactions Local reactions observed during the pivotal clinical study include mild swelling of the site (present in most infusions) due to the large volumes infused, but in general were not considered an adverse reaction unless they caused discomfort. Only two instances of local adverse reactions were severe, infusion site pain and infusion site swelling. There were two instances of transient genital oedema, one considered severe, that resulted from diffusion of the medicinal product from the infusion site in the abdomen. No skin changes were observed that did not resolve during the clinical study. Paediatric population In the pivotal study 160603 there were 2 of the 24 paediatric patients with total anti-rHuPH20 antibody levels at or above 1:160. None had neutralising antibodies. A prospective, Phase 4, multicentre study in Europe evaluated 42 paediatric subjects (age 2 to <18 years) who had received prior immunoglobulin therapy (Study 161504). No new safety concerns were identified. No subject was positive (titre ≥160) for binding antirHuPH20 antibodies. HyQvia was found to be safe and tolerable among paediatric subjects (2 to <18 years old) with PIDD. Results of clinical studies indicate similar safety profiles in adults and paediatric population, including the nature, frequency, seriousness and reversibility of adverse reactions. Elderly Patients Primary Immunodeficiency Post-authorisation safety studies (EU 161302, US 161406) included 15 and 77 elderly subjects, respectively. Overall, no significant safety differences were observed between PID subjects above 65 years and those between 18 and 65 years old. Reporting of suspected adverse reactions Reporting suspected adverse reactions after authorisation of the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the medicinal product. Any suspected adverse events should be reported to the Ministry of Health according to the National Regulation by using an online form https://sideeffects.health.gov.il

פרטי מסגרת הכללה בסל
התרופה תינתן לטיפול במקרים האלה: א. חסר חיסוני ראשוני (חולים עם פגיעה ראשונית בייצור נוגדנים כגון אגמגלובולינמיה או היפוגמגלובוילינמיה, ITP (Idiopathic thrombocytopenic purpura)); ב. חסר חיסוני ספציפי, מניעה או טיפול בחצבת, הפטיטיס A ויראלית; ג. CIDP – Chronic inflammatory demyelineating polyneuropathy; ד.טיפול בחולי לוקמיה מסוג CLL הסובלים מהיפוגלמגלובולינמיה משנית חמורה וזיהומים חוזרים.
שימוש לפי פנקס קופ''ח כללית 1994
Primary immunodeficiency (patients with primary defective antibody synthesis such as agammaglobulinemia or hypogammaglobulinemia, idiopathic thrombocytopenic purpura (ITP)
תאריך הכללה מקורי בסל
01/01/1995
הגבלות
תרופה מוגבלת לשימוש בבתי חולים או אשפוז יום
מידע נוסף
עלון מידע לצרכן
18.11.21 - עלון לצרכן אנגלית 18.11.21 - עלון לצרכן עברית 18.11.21 - עלון לצרכן אנגלית 18.11.21 - עלון לצרכן עברית 15.06.21 - עלון לצרכן אנגלית 15.07.21 - עלון לצרכן עברית 15.06.21 - עלון לצרכן ערבית 06.11.22 - עלון לצרכן אנגלית 06.11.22 - עלון לצרכן עברית 06.11.22 - עלון לצרכן ערבית 12.10.23 - עלון לצרכן אנגלית 12.10.23 - עלון לצרכן עברית 12.10.23 - עלון לצרכן ערבית 14.09.22 - החמרה לעלוןלתרופה במאגר משרד הבריאות
היקיוביה