Quest for the right Drug
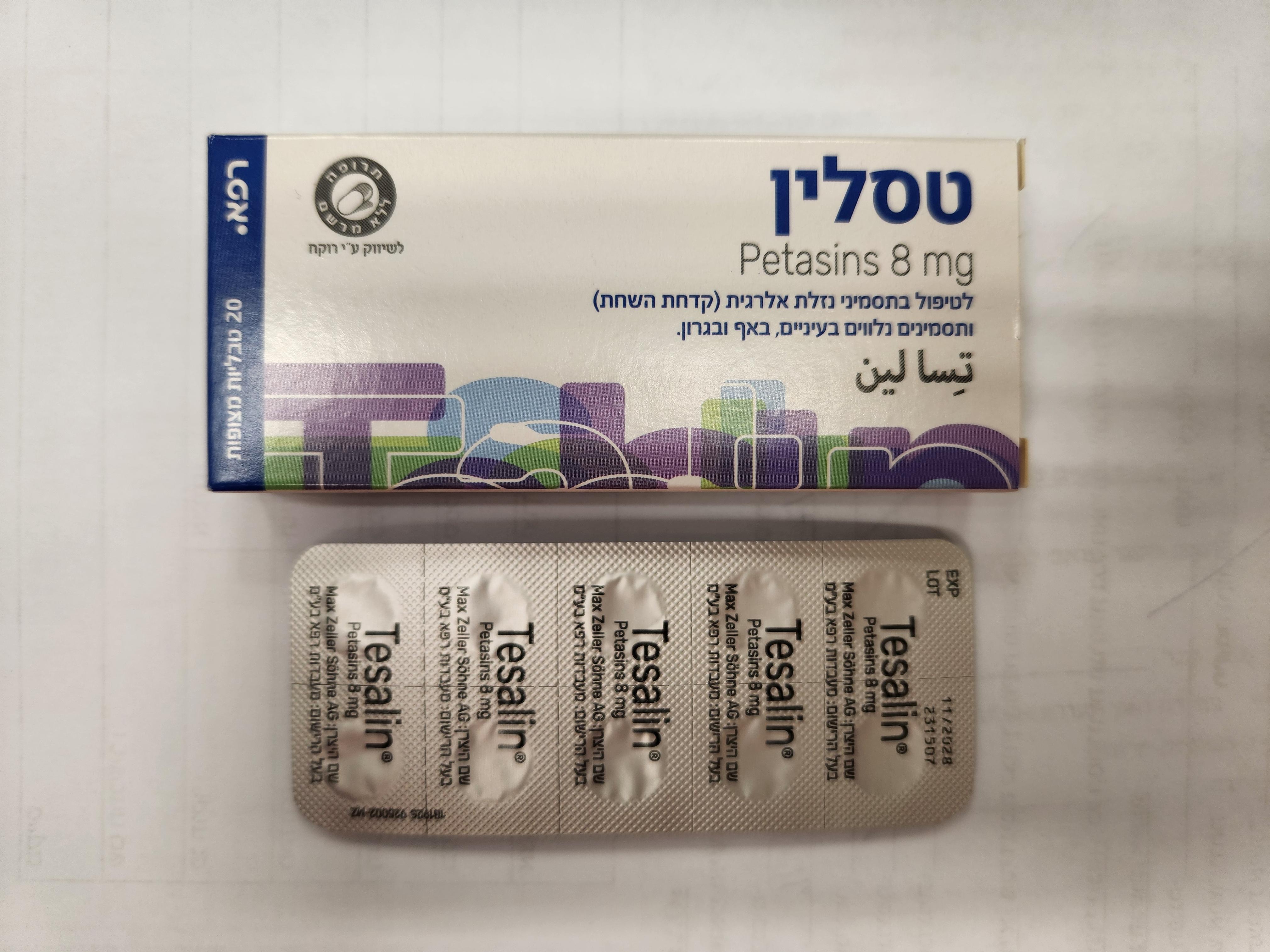
טסלין TESALIN (PETASINS)
תרופה במרשם
תרופה בסל
נרקוטיקה
ציטוטוקסיקה
צורת מתן:
פומי : PER OS
צורת מינון:
טבליה : TABLETS
עלון לרופא
מינוניםPosology התוויות
Indications תופעות לוואי
Adverse reactions התוויות נגד
Contraindications אינטראקציות
Interactions מינון יתר
Overdose הריון/הנקה
Pregnancy & Lactation אוכלוסיות מיוחדות
Special populations תכונות פרמקולוגיות
Pharmacological properties מידע רוקחי
Pharmaceutical particulars אזהרת שימוש
Special Warning עלון לרופא
Physicians Leaflet
Pharmacological properties : תכונות פרמקולוגיות
Pharmacodynamic Properties
5.1 Pharmacodynamic properties Pharmacotherapeutic group: Other respiratory system products, ATC code: R07AX. In an explorative study with 18 symptom-free adult patients with a history of allergic rhinitis caused by grass pollen, a faster reduction of nasal obstruction after targeted provocation with grass pollen could be shown under treatment with Tesalin compared to placebo and desloratadine. Clinical efficacy: The efficacy of Tesalin in allergic rhinitis has been studied so far in 3 clinical trials. In a three-arm study, the superiority of 2 and 3 film coated tablets Tesalin versus placebo was demonstrated for the TSS (total sum score) as well as for the improvement of individual symptoms, such as sneezing, itchy nose and eyes, rhinorrhoea and nasal obstruction. When using 3 film coated tablets Tesalin, 91% responders (≥ 25% improvement of TSS) and with 2 film coated tablets 71% responders were observed. In an additional trial Tesalin and fexofenadine were tested for their efficacy in comparison to placebo. Both active ingredients significantly improved the TSS, as well as the individual symptoms sneezing, itchy nose, itchy/reddened eyes and rhinorrhoea. In a third trial the effects of Tesalin and cetirizine with respect to the patients’ quality of life were determined. An equivalent efficacy (non-inferiority) of Tesalin and cetirizine resulted.
Pharmacokinetic Properties
5.2 Pharmacokinetic properties In a kinetic phase-I-study with crossover design, 24 male healthy volunteers were treated with single administration of either 2 or 4 tablets containing P. hybridus extract (CO2 extract Ze 339 from leaves of Petasites hybridus (L.) Gaertn., B. Mey. et Scherb.). The average dosage of measured petasin corresponded to 0.20 mg/ kg body weight for 2 tablets or 0.41 mg/ kg body weight for 4 tablets. Absorption No corresponding studies were conducted. Distribution The following data are available for the ingredient petasin, but no conclusions can be drawn for distribution of the total extract, i.e. the active substance. Maximal plasma concentrations (cmax) were reached after approx. 1.6 hours (tmax) dose- independently for both dosages (SD ± 0.499 or ± 0.926, respectively) and were dose-dependend at 25.5 ± 14.8 ng/ ml after administration of 2 tablets and at 58.1 ± 26.7 ng/ ml after administration of 4 tablets; the AUC was proportional to the dose at 65.3 ± 35.61 ng/ ml*h after administration of 2 tablets and at 151.2 ± 68.21 ng/ ml*h after administration of 4 tablets. Metabolism No corresponding studies were conducted. Elimination Elimination of petasin was comparable between the two doses, with a half life time of 7.155 ± 4.611 h (2 tablets) and 7.618 ± 3.338 h (4 tablets).

שימוש לפי פנקס קופ''ח כללית 1994
לא צוין
תאריך הכללה מקורי בסל
לא צוין
הגבלות
לא צוין
מידע נוסף
