Quest for the right Drug
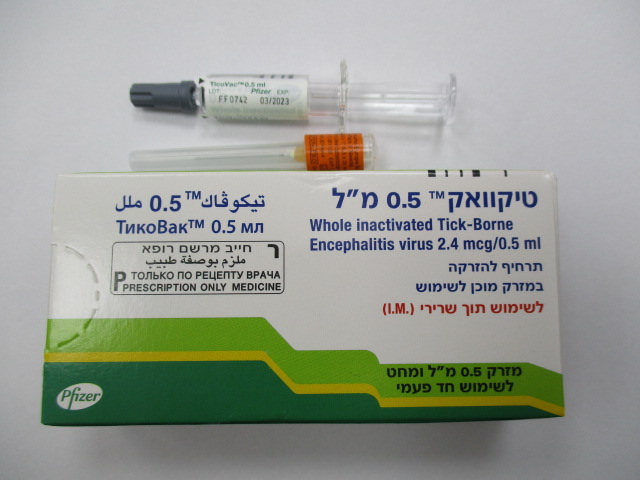
טיקוואק 0.5 מ"ל TICOVAC 0.5 ML (ENCEPHALITIS, TICK BORNE, INACTIVATED, WHOLE VIRUS)
תרופה במרשם
תרופה בסל
נרקוטיקה
ציטוטוקסיקה
צורת מתן:
תוך-שרירי : I.M
צורת מינון:
תרחיף להזרקה : SUSPENSION FOR INJECTION
עלון לרופא
מינוניםPosology התוויות
Indications תופעות לוואי
Adverse reactions התוויות נגד
Contraindications אינטראקציות
Interactions מינון יתר
Overdose הריון/הנקה
Pregnancy & Lactation אוכלוסיות מיוחדות
Special populations תכונות פרמקולוגיות
Pharmacological properties מידע רוקחי
Pharmaceutical particulars אזהרת שימוש
Special Warning עלון לרופא
Physicians Leaflet
Adverse reactions : תופעות לוואי
4.8 Undesirable effects The frequencies provided in the table below are per vaccination and have been calculated based on a pooled analysis of adverse reactions from 7 clinical studies conducted with TicoVac 0.5 mL (2.4 µg) in subjects aged 16 through 65 years receiving 3 vaccinations (3512 subjects after the first vaccination, 3477 after the second vaccination, and 3274 after the third vaccination). The ADRs listed in this section are given according to the recommended frequency convention: Adverse Reactions from clinical trials System organ class Frequency Very common Common Uncommon Rare (1/10) (1/100 to (1/1,000 to <1/100) (1/10,000 to <1/10) <1/1,000) Blood and lymphatic Lymphadenopathy system disorders Immune system Hypersensitivity disorders Nervous system Headache Somnolence disorders Ear and labyrinth Vertigo1 disorders Gastrointestinal Nausea Vomiting Diarrhoea disorders Abdominal pain Musculoskeletal and Myalgia connective tissue Arthralgia disorders General disorders Injection site Fatigue Pyrexia Injection site reactions and administration reactions Malaise Injection site such as site conditions e.g., Injection site hemorrhage • Erythema pain • Induration • Swelling • Pruritus • Paraesthesia • Warmth Adverse reactions from post-marketing surveillance 1 The frequency of vertigo is based on the rate reported after the first vaccination (n=3512). Vertigo was not reported after the second or third vaccinations. The following additional adverse reactions have been reported in post-marketing experience. System organ class Frequency* Rare (1/10,000 to <1/1,000) Infections and infestations Herpes zoster (triggered in pre-exposed patients) Immune system disorders Precipitation or aggravation of autoimmune disorders (e.g. multiple sclerosis), anaphylactic reaction Nervous system disorders Demyelinating disorders (acute disseminated encephalomyelitis, guillain-barré syndrome, myelitis, transverse myelitis), encephalitis, convulsions, aseptic meningitis, meningism, sensory abnormalities and motor dysfunction (facial palsy/paresis, paralysis/paresis, neuritis, hypoesthesia, paresthesia), neuralgia, optic neuritis, dizziness Eye disorders Visual impairment, photophobia, eye pain Ear and labyrinth disorders Tinnitus Cardiac disorders Tachycardia Respiratory, thoracic and mediastinal disorders Dyspnea Skin and subcutaneous tissue disorders Urticaria, rash (erythematous, maculopapular), pruritus, dermatitis, erythema, hyperhidrosis Musculoskeletal and connective tissue disorders Back pain, joint swelling, neck pain, musculoskeletal stiffness (including neck stiffness), pain in extremity General disorders and administration site Gait disturbance, chills, influenza-like illness, asthenia, edema, conditions injection site joint movement impairment such as joint pain, nodule and inflammation * The upper limit of the 95% confidence interval of the event frequency is calculated with 3/n, with n representing the number of subjects included in all clinical trials with TicoVac 0.5 ml. Therefore, the calculated frequency “rare” represents the theoretical maximum frequency for these events In a small comparative study on the immune response after intramuscular and subcutaneous administration of TicoVac in healthy adults, the subcutaneous route led to a higher local reactogenicity profile, particularly in women. Reporting of suspected adverse reactions Reporting suspected adverse reactions after authorisation of the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the medicinal product. Any suspected adverse events should be reported to the Ministry of Health according to the National Regulation by using an online form https://sideeffects.health.gov.il/

שימוש לפי פנקס קופ''ח כללית 1994
לא צוין
תאריך הכללה מקורי בסל
לא צוין
הגבלות
לא צוין
מידע נוסף
