Quest for the right Drug
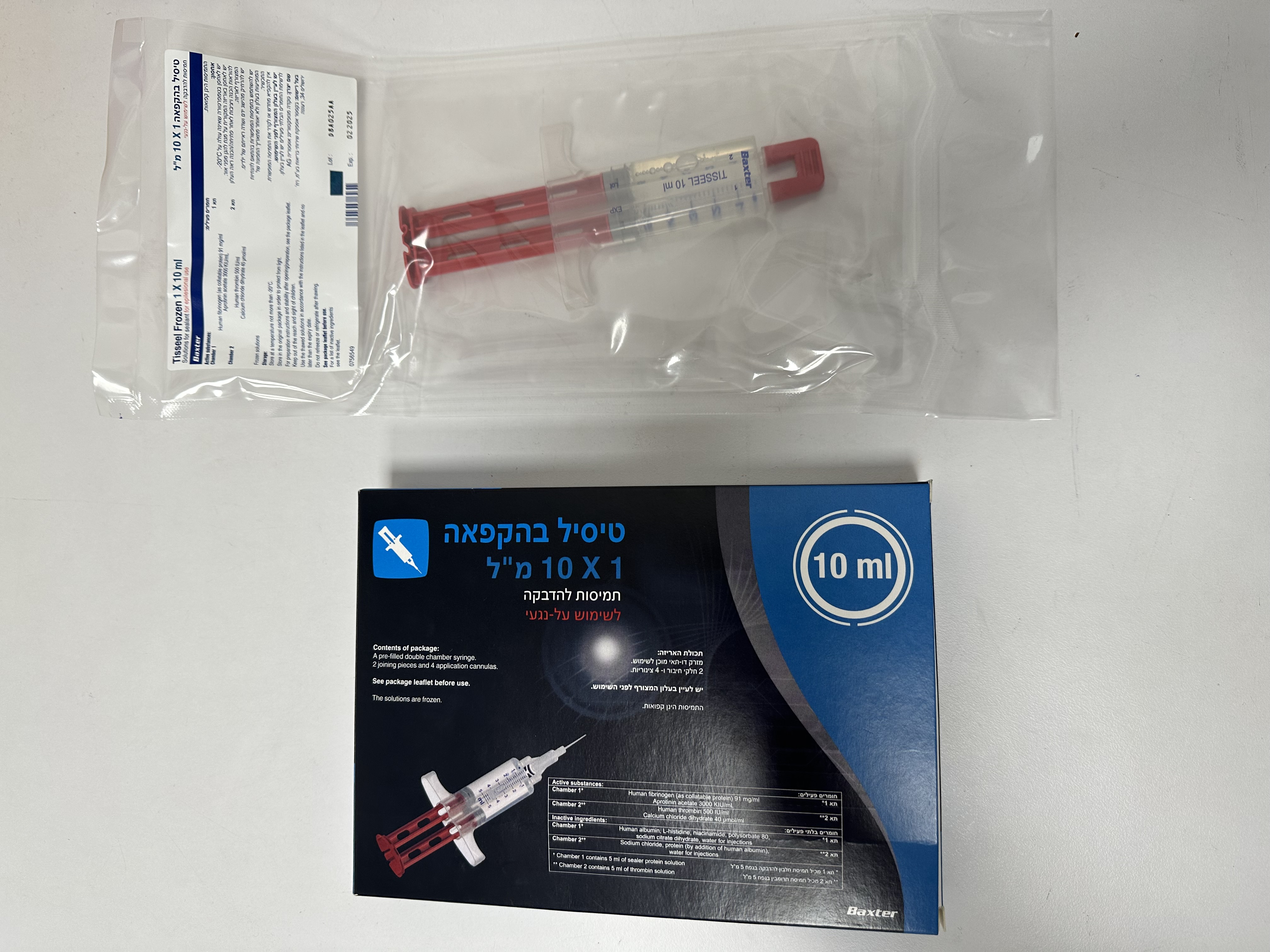
טיסיל בהקפאה TISSEEL FROZEN (APROTININ ACETATE, CALCIUM CHLORIDE DIHYDRATE, HUMAN FIBRINOGEN, HUMAN THROMBIN)
תרופה במרשם
תרופה בסל
נרקוטיקה
ציטוטוקסיקה
צורת מתן:
לסביבת הפצע : EPILESIONAL
צורת מינון:
אין פרטים : SOLUTIONS FOR SEALANT
עלון לרופא
מינוניםPosology התוויות
Indications תופעות לוואי
Adverse reactions התוויות נגד
Contraindications אינטראקציות
Interactions מינון יתר
Overdose הריון/הנקה
Pregnancy & Lactation אוכלוסיות מיוחדות
Special populations תכונות פרמקולוגיות
Pharmacological properties מידע רוקחי
Pharmaceutical particulars אזהרת שימוש
Special Warning עלון לרופא
Physicians Leaflet
Adverse reactions : תופעות לוואי
4.8 Undesirable effects Hypersensitivity or allergic reactions (which may include but are not limited to angioedema, burning and stinging at the application site, bradycardia, bronchospasm, chills, breathing difficulties, transient erythema (“flushing”), generalized urticaria, headache, hives, hypotension, lethargy, nausea, pruritus, restlessness, paresthesia, tachycardia, tightness of the chest, tingling, vomiting, wheezing) may occur in rare cases in patients treated with fibrin sealants / hemostatics, anaphylactic reactions and anaphylactic shock have included fatal outcomes. In isolated cases, these reactions have progressed to severe anaphylaxis. Such reactions may especially be seen if the preparation is applied repeatedly or administered to patients known to be hypersensitive to aprotinin (see section 4.4) or any other constituents of the product. Even if repeated treatment with TISSEEL FROZEN was well tolerated, a subsequent administration of TISSEEL FROZEN or systemic administration of aprotinin may result in severe anaphylactic reactions. Antibodies against components of the fibrin sealant / hemostatic may occur in rare cases. Inadvertent intravascular injection may result in thromboembolic events and DIC. Furthermore there is the risk of an anaphylactic reaction (see section 4.4). For safety with respect to transmissible agents, see section 4.4. The adverse reactions presented in this section were reported from clinical trials investigating the safety and efficacy of TISSEEL FROZEN and from post-marketing experience (marked with a p in the adverse event table below) with Baxter Fibrin Sealants. In the clinical trials, TISSEEL FROZEN was administered for adjunct hemostasis in cardiac, vascular, and total hip replacement surgeries and in liver and spleen surgeries. Other clinical trials included the sealing of lymphatic vessels in patients undergoing axillary lymph node dissection, sealing of colonic anastomosis and in dural sealing in the posterior fossa. As the frequencies of adverse events observed in post marketing experience cannot be calculated, whenever possible, the upper limit of the 95% confidence interval was calculated using the “rule of three” in the following manner: 3/1146 = 0.0026 or 0.26% which is “Uncommon” (where “1146” is the total number of subjects to have received TISSEEL FROZEN in the clinical trials from which data were included in the SmPC) Very common (≥ 1/10) Common (≥ 1/100 to <1/10) Uncommon (≥ 1/1,000 to <1/100) Rare (≥ 1/10,000 to <1/1,000) Very rare (< 1/10,000) Not known (cannot be estimated from the available data) System organ class Preferred MedDRA Term Frequency (SOC) Infections and Postoperative wound infection Common infestations Blood and lymphatic Fibrin degradation Uncommon system disorders products increased Immune system Hypersensitivity reactions* p Uncommon disorders Anaphylactic reactions* p Uncommon Anaphylactic shock* p Uncommon Paresthesia p Uncommon Bronchospasm p Uncommon Wheezing p Uncommon Pruritus p Uncommon Erythema p Uncommon Nervous system Sensory disturbance Common disorders Cardiac disorders Bradycardia p Uncommon Tachycardia p Uncommon Vascular disorders Axillary vein thrombosis ** Common Hypotension Rare Haematoma (NOS) p Uncommon Embolism arterial p Uncommon Air embolism*** p Not known Cerebral artery embolism p Uncommon Cerebral infarction** p Uncommon Respiratory, thoracic Dyspnoea p Uncommon and mediastinal disorders Gastrointestinal Nausea Uncommon p disorders Intestinal obstruction Uncommon Skin and subcutaneous Rash Common tissue disorders Urticaria p Uncommon Impaired healing p Uncommon Musculoskeletal and Pain in an extremity Common connective tissue disorders General disorders and Pain Common administration site Increased body temperature Common conditions Flushing p Uncommon Oedema p Uncommon Injury, poisoning and Procedural pain Uncommon procedural Seroma Very common complications Angioedema p Uncommon * anaphylactic reactions and anaphylactic shock have included fatal outcomes. ** as a result of intravascular application into the superior petrosal sinus. *** as with other fibrin sealants life-threatening/fatal air or gas embolism when using devices with pressurized air or gas occurred; this event appears to be related to an inappropriate use of the spray device (e.g. at higher than recommended pressure and in close proximity to the tissue surface). p Adverse events observed in post-marketing experience. Class Reactions Other adverse reactions associated with the fibrin sealant/hemostatic class include: manifestations of hypersensitivity include application site irritation, chest discomfort, chills, headache, lethargy, restlessness, and vomiting. Reporting of suspected adverse reactions Reporting suspected adverse reactions after authorisation of the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the medicinal product. Any suspected adverse events should be reported to the Ministry of Health according to the National Regulation by using an online form: https://sideeffects.health.gov.il

שימוש לפי פנקס קופ''ח כללית 1994
לא צוין
תאריך הכללה מקורי בסל
לא צוין
הגבלות
לא צוין
רישום
162 87 35322 00
מחיר
0 ₪
מידע נוסף
