Quest for the right Drug
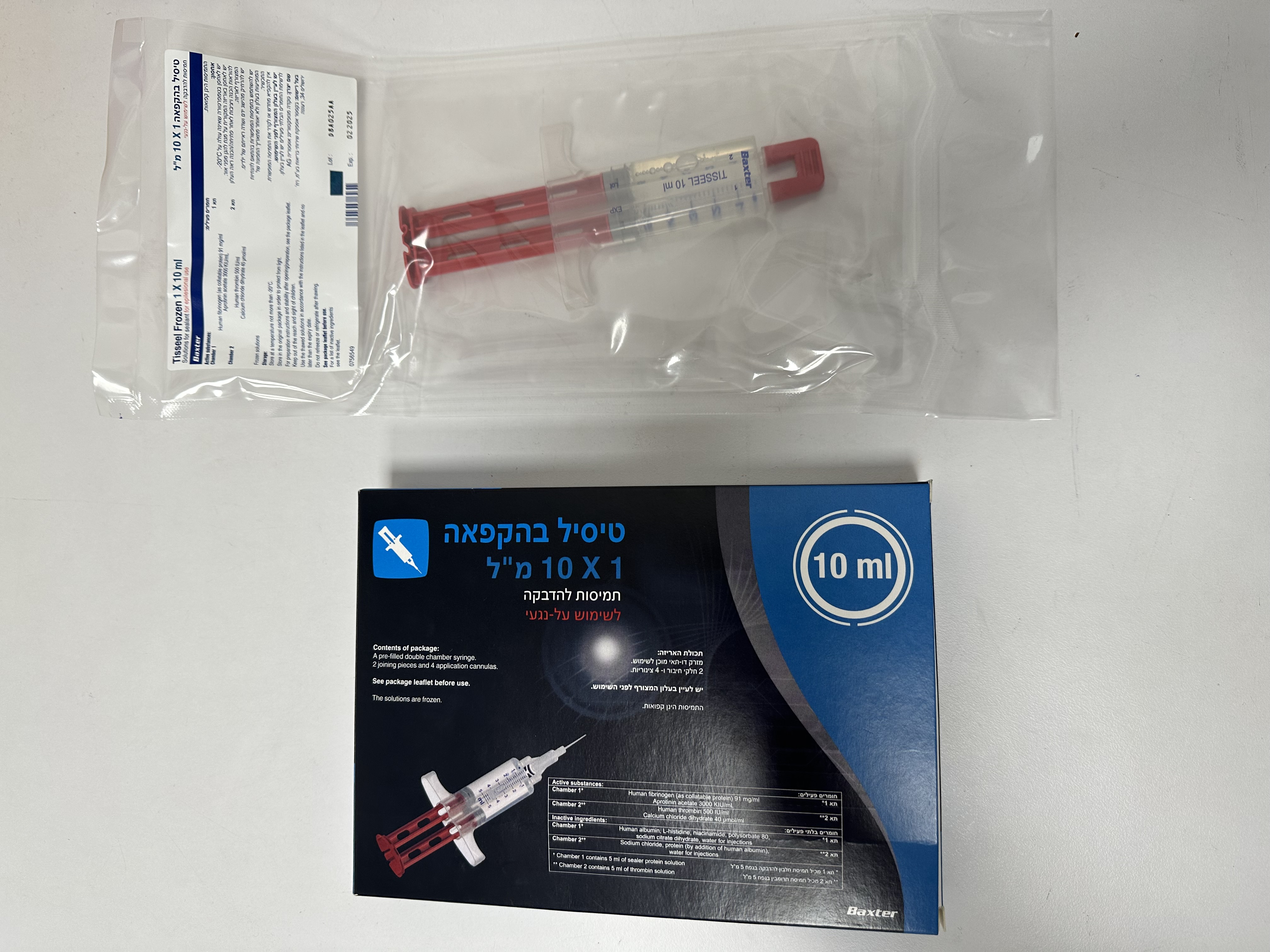
טיסיל בהקפאה TISSEEL FROZEN (APROTININ ACETATE, CALCIUM CHLORIDE DIHYDRATE, HUMAN FIBRINOGEN, HUMAN THROMBIN)
תרופה במרשם
תרופה בסל
נרקוטיקה
ציטוטוקסיקה
צורת מתן:
לסביבת הפצע : EPILESIONAL
צורת מינון:
אין פרטים : SOLUTIONS FOR SEALANT
עלון לרופא
מינוניםPosology התוויות
Indications תופעות לוואי
Adverse reactions התוויות נגד
Contraindications אינטראקציות
Interactions מינון יתר
Overdose הריון/הנקה
Pregnancy & Lactation אוכלוסיות מיוחדות
Special populations תכונות פרמקולוגיות
Pharmacological properties מידע רוקחי
Pharmaceutical particulars אזהרת שימוש
Special Warning עלון לרופא
Physicians Leaflet
Pharmaceutical particulars : מידע רוקחי
6. PHARMACEUTICAL PARTICULARS 6.1 List of excipients Component 1: Sealer protein solution: L-histidine Human albumin Sodium citrate dihydrate Niacinamide Polysorbate 80 Water for Injections Component 2: Thrombin solution: Protein (by addition of human albumin) Sodium chloride Water for Injections 6.2 Incompatibilities Oxidized cellulose-containing preparations should not be used with TISSEEL FROZEN because the low pH interferes with the activity of the thrombin. This medicinal product must not be mixed with other medicinal products except those mentioned in section 6.6. 6.3 Shelf life The expiry date of the product is indicated on the packaging materials. 6.4 Special precautions for storage Store at a temperature not more than -20°C. Store in the original package in order to protect from light. After thawing: after thawing and warming (at temperatures between 33°C and 37°C), chemical and physical product stability has been demonstrated for 12 hours at 33°C to 37°C. For product thawed at up to 25°C in the unopened bag, chemical and physical product stability has been demonstrated for 72 hours at temperatures not more than 25°C. From a microbiological point of view, unless the method of opening/thawing precludes the risks of microbial contamination, the product should be used immediately after being warmed to 33°C to 37°C. If not used immediately, in-use storage times and conditions are the responsibility of the user. Do not re-freeze or refrigerate after thawing. 6.5 Nature and contents of container Content of package with PRIMA Syringe: - 1 ml, 2 ml or 5 ml sealer protein solution and 1 ml, 2 ml or 5 ml thrombin solution in a pre-filled double chamber syringe (polypropylene) closed with a tip cap packed in two bags and with a device with 2 joining pieces and 4 applications cannulas. Pack sizes: TISSEEL FROZEN is available in the following pack sizes: 1 x 2 ml (1 ml + 1 ml), 1 x 4 ml (2 ml + 2 ml) and 1 x 10 ml (5 ml + 5 ml). Not all pack sizes may be marketed. Other accessories for application of the product can be obtained from BAXTER. 6.6 Special precautions for disposal and other handling General • Before the administration of TISSEEL FROZEN, cover all parts of the body outside the area to be treated in order to prevent tissue adhesion at undesired sites. • To prevent TISSEEL FROZEN from adhering to gloves and instruments, wet these with sodium chloride solution before contact. • The guideline for sealing surfaces is: One package of TISSEEL FROZEN 2 ml (i.e. 1 ml sealer protein solution plus 1 ml thrombin solution) is sufficient for a surface of at least 10 cm2. • The dose required depends on the size of the surface to be sealed. • Do NOT apply the two components of TISSEEL FROZEN separately. Both components must be applied together. • Do NOT expose TISSEEL FROZEN to temperatures above 37°C. Do NOT microwave. • Do NOT thaw the product by holding it in your hands. • Do NOT use TISSEEL FROZEN until it is completely thawed and warmed to 33°C – 37°C. • Remove the protective cap of the syringe only when thawing and warming is complete. For PRIMA syringe: To facilitate removal of the tip cap from the syringe, rock the tip cap by moving it backward and forward, then pull the protective cap off the syringe. • Expel all air from the syringe then attach the joining piece and application cannula. Instructions for handling and preparation: Both the sealer protein solution and the thrombin solution are contained in a ready-to-use syringe. The product is packed in two sterile bags under aseptic conditions. The inner bag and its contents are sterile as long as the outer bag remains intact. Using sterile technique, transfer the sterile inner pouch and contents onto the sterile field. The ready-to-use syringe may be thawed AND warmed using one of the following methods: 1. Rapid thawing/warming (sterile water bath) – Recommended method 2. Thawing/warming in a non-sterile water bath 3. Thawing/warming in an incubator 4. The ready-to-use syringe may also be thawed and kept at room temperature (not above 25°C) for up to 72 hours. Warming is required prior to use. 1) Rapid thawing/warming (sterile water bath) – Recommended method It is recommended to thaw and warm the two sealant components using a sterile water bath at a temperature of 33 – 37°C. • The water bath must not exceed a temperature of 37°C. In order to monitor the specified temperature range, control the water temperature using a thermometer and change the water as necessary. • When using a sterile water bath for thawing and warming, remove the pre-filled syringe from the bags before placing it in the sterile water bath. Instructions: Bring the inner bag into the sterile area, remove the ready-to-use syringe from the inner bag and place it directly in the sterile water bath. Ensure that the content of the ready-to- use syringe is completely immersed in the water. Table 1: Minimum thawing and warming times using a sterile water bath Pack Minimum Thawing/Warming Times Size 33°C to 37°C, Sterile Water Bath Product without bags PRIMA Syringe 2 ml 5 minutes 4 ml 5 minutes 10 ml 10 minutes 2) Thawing/warming in a non-sterile water bath Instructions: Leave the ready-to-use syringe inside both bags and place it in a water bath outside the sterile area for the appropriate length of time (see Table 2). Ensure that the bags remain immersed in the water during the entire thawing time. After thawing, remove the bags from the water bath, dry the outer bag and bring the inner bag with the ready-to-use syringe into the sterile area. Table 2: Minimum thawing and warming times using a non-sterile water bath Pack Minimum Thawing/Warming Times Size 33°C to 37°C, Non-sterile Water Bath Product in bags PRIMA Syringe 2 ml 15 minutes 4 ml 20 minutes 10 ml 35 minutes 3) Thawing/warming in an incubator Instructions: Leave the ready-to-use syringe inside both bags and place it in an incubator outside the sterile area for the appropriate length of time (see Table 3). After thawing/warming, remove the bags from the incubator, remove the outer bag and bring the inner bag with the ready-to-use syringe into the sterile area. Table 3: Minimum thawing and warming times in an incubator Pack Minimum Thawing/Warming Times Size 33°C to 37°C, Incubator Product in bags PRIMA Syringe 2 ml 40 minutes 4 ml 50 minutes 10 ml 90 minutes 4) Thawing at room temperature (not above 25°C) BEFORE warming Instructions: Leave the ready-to-use syringe inside both bags and thaw it at room temperature outside the sterile area for the appropriate length of time (see Table 4). Once thawed, in order to warm the product for use, warm it in the outer bag in an incubator. Table 4: Minimum thawing times at room temperature outside of the sterile field and additional warming times in an incubator to 33°C to 37°C Minimum Thawing Times of product at room temperature (not above 25°C) followed by additional warming, prior to use, in an incubator at Pack 33°C to a maximum of 37°C Size Product in bags PRIMA Syringe Thawing at room Warming in Incubator temperature (33-37°C) (not above 25°C) +11 2 ml 80 minutes minutes +13 4 ml 90 minutes minutes +25 10 ml 160 minutes minutes After thawing at room temperature, the product must be used within 72 hours of removing from the freezer. Stability after thawing After thawing and warming (at temperatures between 33°C and 37°C, methods 1, 2 and 3), chemical and physical product stability has been demonstrated for 12 hours at 33°C to 37°C. For product thawed at room temperature in the unopened bag (method 4), chemical and physical product stability has been demonstrated for 72 hours at temperatures no more than 25°C. Warm to 33°C to 37°C immediately before use. From a microbiological point of view, unless the method of opening/thawing precludes the risks of microbial contamination, the product should be used immediately after being warmed to 33°C to 37°C. If not used immediately, in-use storage times and conditions are the responsibility of the user. Do not re-freeze or refrigerate once thawing has been initiated. Handling after thawing / before application To achieve optimal blending of the two solutions and optimal solidification of the fibrin sealant, maintain the two sealant components at 33°C - 37°C until application. The sealer protein and the thrombin solutions should be clear or slightly opalescent. Do not use solutions that are cloudy or have deposits. Before use, check the thawed product visually for particles, discoloration or other changes in its appearance. If one of the above occurs, dispose of the solutions. The thawed sealer protein solution should be liquid but slightly viscous. If the solution has the consistency of a solidified gel, it must be assumed to have become denatured (possibly due to an interruption of the cold storage chain or by overheating during warming). In this case, do NOT use TISSEEL FROZEN on any account. • Remove the syringe from the bags shortly before use. • Use TISSEEL FROZEN only when it is thawed and warmed completely (liquid consistency). • Remove the protective cap from the syringe immediately before application. For PRIMA syringe: To facilitate removal of the tip cap from the syringe, rock the tip cap by moving it backward and forward, then pull the protective cap off the syringe. Administration with PRIMA Syringe: For application, connect the double chamber ready-to-use syringe with the sealer protein solution and the thrombin solution to a joining piece and an application cannula – both are provided in the set with the application devices. The common plunger of the double chamber ready-to-use syringe ensures that equal volumes of the two sealant components are fed through the joining piece into the application cannula where they are blended and then applied. Operating instructions for PRIMA Syringe: Tether Strap Double Plunger Double Chamber Syringe Joining piece Application Cannula • Expel all air from the syringe prior to attaching any application device. • Align the joining piece and tether to the side of the syringe with the tether strap hole. • Connect the nozzles of the double chamber ready-to-use syringe to the joining piece, ensuring that they are firmly attached. o Secure the joining piece by fastening the tether strap to the double chamber ready-to-use syringe. o If the tether strap tears, use the spare joining piece provided in the kit. o If a spare joining piece is not available, the system can still be used if care is taken to ensure that the connection is secure and leak-proof. o Do NOT expel the air remaining inside the joining piece. • Attach an application cannula on to the joining piece. o Do NOT expel the air remaining inside the joining piece and inside the application cannula until you start the actual application because this may clog the application cannula. Administration Prior to applying TISSEEL FROZEN the surface of the wound needs to be dried by standard techniques (e.g. intermittent application of compresses, swabs, use of suction devices). Do not use pressurized air or gas for drying the site. • Apply the mixed sealer protein - thrombin solution on to the recipient surface or on to the surfaces of the parts to be glued by slowly pressing on the back of the common plunger. • In surgical procedures that require the use of minimal volumes of fibrin sealant, it is recommended to expel and discard the first few drops of product. • After TISSEEL FROZEN has been applied, allow at least 2 minutes to achieve sufficient polymerization. Spray application When applying TISSEEL FROZEN using a spray device be sure to use a pressure and a distance from tissue within the ranges recommended by the manufacturer as follows: Recommended pressure, distance and devices for spray application of TISSEEL FROZEN Recommend Spray set to be Applicator tips to be Pressure regulator ed distance Recommended Surgery used used to be used from target spray pressure tissue Tisseel / Artiss n.a. EasySpray Spray Set 1.5-2.0 bar Open wound Tisseel / Artiss 10 - 15 cm (21.5-28.5 psi) Spray Set 10 n.a. EasySpray pack Duplospray MIS Applicator 20cm Duplospray MIS Applicator 30cm Duplospray MIS Applicator 40cm Laparoscopic/ Spray Set 360 Duplospray MIS minimally Endoscopic 1.2-1.5 bar n.a. Regulator 1.5 bar 2 - 5 cm invasive Applicator with (18-22 psi) procedures Snap Lock Spray Set 360 Endoscopic Applicator with Tether Replaceable tip When spraying TISSEEL FROZEN, changes in blood pressure, pulse, oxygen saturation and end tidal CO2 should be monitored because of the possibility of occurrence of air or gas embolism (see sections 4.2 and 4.4). For the application of TISSEEL FROZEN in enclosed thoracic and abdominal spaces the DuploSpray MIS applicator and regulator system is recommended. Please refer to the instruction manual of the DuploSpray MIS device. Disposal Any unused medicinal product or waste material should be disposed of in accordance with local requirements. 7. REGISTRATION NUMBER 162-87-35322

שימוש לפי פנקס קופ''ח כללית 1994
לא צוין
תאריך הכללה מקורי בסל
לא צוין
הגבלות
לא צוין
רישום
162 87 35322 00
מחיר
0 ₪
מידע נוסף
