Quest for the right Drug
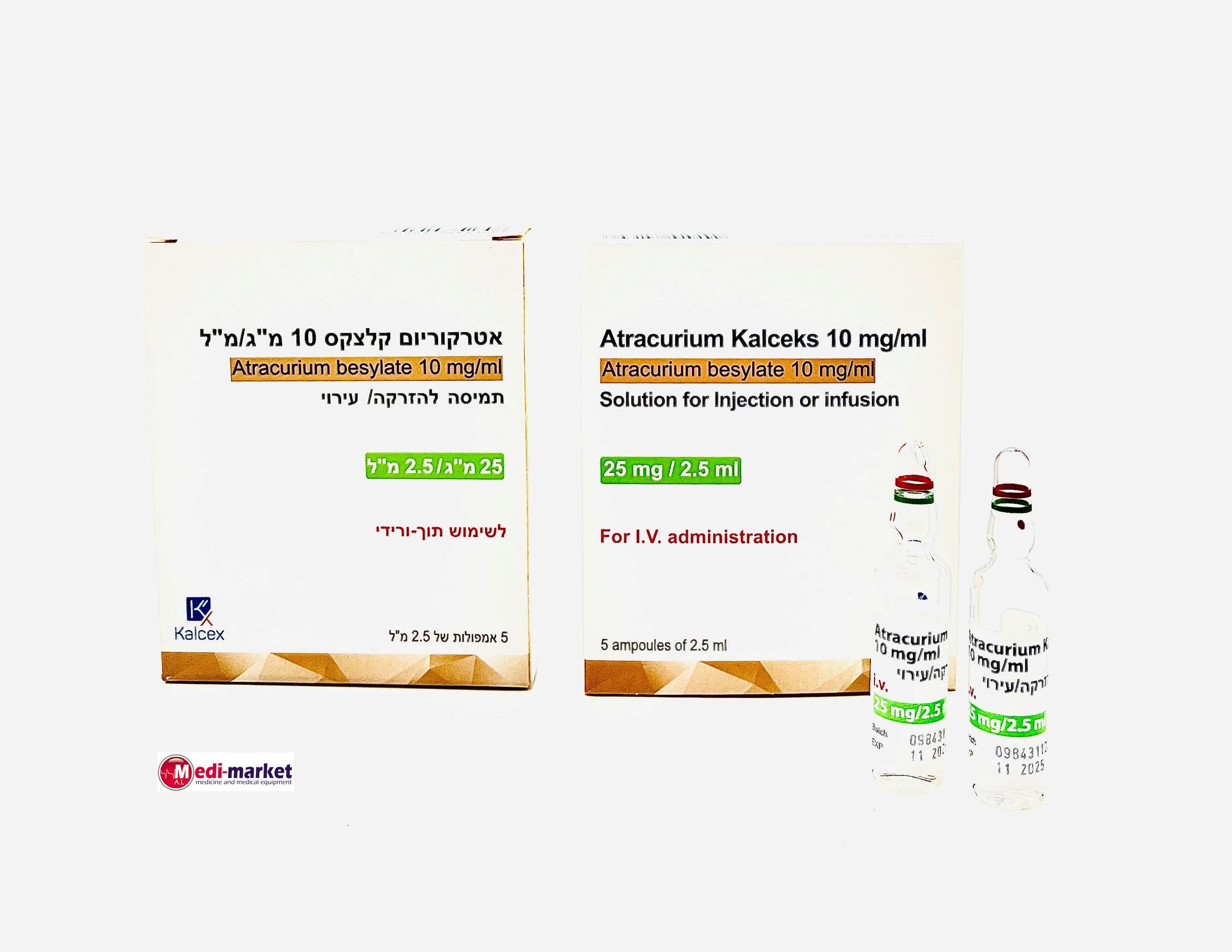
אטרקוריום קלצקס 10 מ"ג/מ"ל ATRACURIUM KALCEKS 10 MG/ML (ATRACURIUM BESYLATE)
תרופה במרשם
תרופה בסל
נרקוטיקה
ציטוטוקסיקה
צורת מתן:
תוך-ורידי : I.V
צורת מינון:
תמיסה להזרקהאינפוזיה : SOLUTION FOR INJECTION / INFUSION
עלון לרופא
מינוניםPosology התוויות
Indications תופעות לוואי
Adverse reactions התוויות נגד
Contraindications אינטראקציות
Interactions מינון יתר
Overdose הריון/הנקה
Pregnancy & Lactation אוכלוסיות מיוחדות
Special populations תכונות פרמקולוגיות
Pharmacological properties מידע רוקחי
Pharmaceutical particulars אזהרת שימוש
Special Warning עלון לרופא
Physicians Leaflet
Pharmaceutical particulars : מידע רוקחי
6. PHARMACEUTICAL PARTICULARS 6.1 List of excipients Benzenesulfonic acid (for pH adjustment) Water for injections 6.2 Incompatibilities Atracurium besilate is inactivated by high pH, thus it must not be mixed in the same syringe with alkaline solutions (e.g. solutions of thiopental). This medicine is a hypotonic solution, thus it must not be administered into the same venous access as a blood transfusion. This medicinal product must not be mixed with other medicinal products except those mentioned in section 6.6. 6.3 Shelf life Shelf life before first opening: The expiry date of the product is indicated on the Packaging materials. For single use only. Once opened, the product should be used immediately. Shelf life after dilution: Chemical and physical in-use stability has been demonstrated in 0.9% NaCl Infusion solution for 24 hours at 25°C, in 5% glucose, Ringer and 0.18% NaCl/ 4% glucose infusion solutions for 8 hours at 25°C, and in Ringer lactate infusion solution for 4 hours at 25°C. From a microbiological point of view, unless the method of dilution precludes the risk of microbial contamination, the product should be used immediately. If not used immediately, in-use storage times and conditions are the responsibility of the user. 6.4 Special precautions for storage Store in a refrigerator (2°C – 8°C). Do not freeze. Store in the original package in order to protect from light. For storage conditions after first opening of the medicinal product, see section 6.3. For storage conditions after dilution of the medicinal product, see section 6.3 and 6.6. 6.5 Nature and contents of container 2.5 ml or 5.0 ml of solution filled in 5.0 ml type I colourless borosilicate glass ampoules with break line or one point cut. Pack size: 5 ampoules. Not all pack sizes may be marketed. 6.6 Special precautions for disposal The solution is to be visually inspected prior to use. Only clear solutions practically free from particles should be used. Atracurium besilate is compatible with the following infusion solutions for the times stated below: Infusion solution Period of stability Sodium chloride intravenous infusion (9 24 hours mg/ml) Glucose intravenous infusion (50 mg/ml) 8 hours Ringer intravenous infusion 8 hours Sodium chloride (1.8 mg/ml) and glucose 8 hours (40 mg/ml) intravenous infusion Ringer lactate intravenous infusion 4 hours When diluted in these solutions to give atracurium besilate concentrations of 0.5 mg/ml and above, the resultant solutions will be stable in daylight for the stated periods at temperatures of up to 25°C. Any unused medicinal product or waste material should be disposed of in accordance with local requirements.

שימוש לפי פנקס קופ''ח כללית 1994
לא צוין
תאריך הכללה מקורי בסל
01/01/1995
הגבלות
תרופה מוגבלת לשימוש בבתי חולים או אשפוז יום
מידע נוסף
