Quest for the right Drug
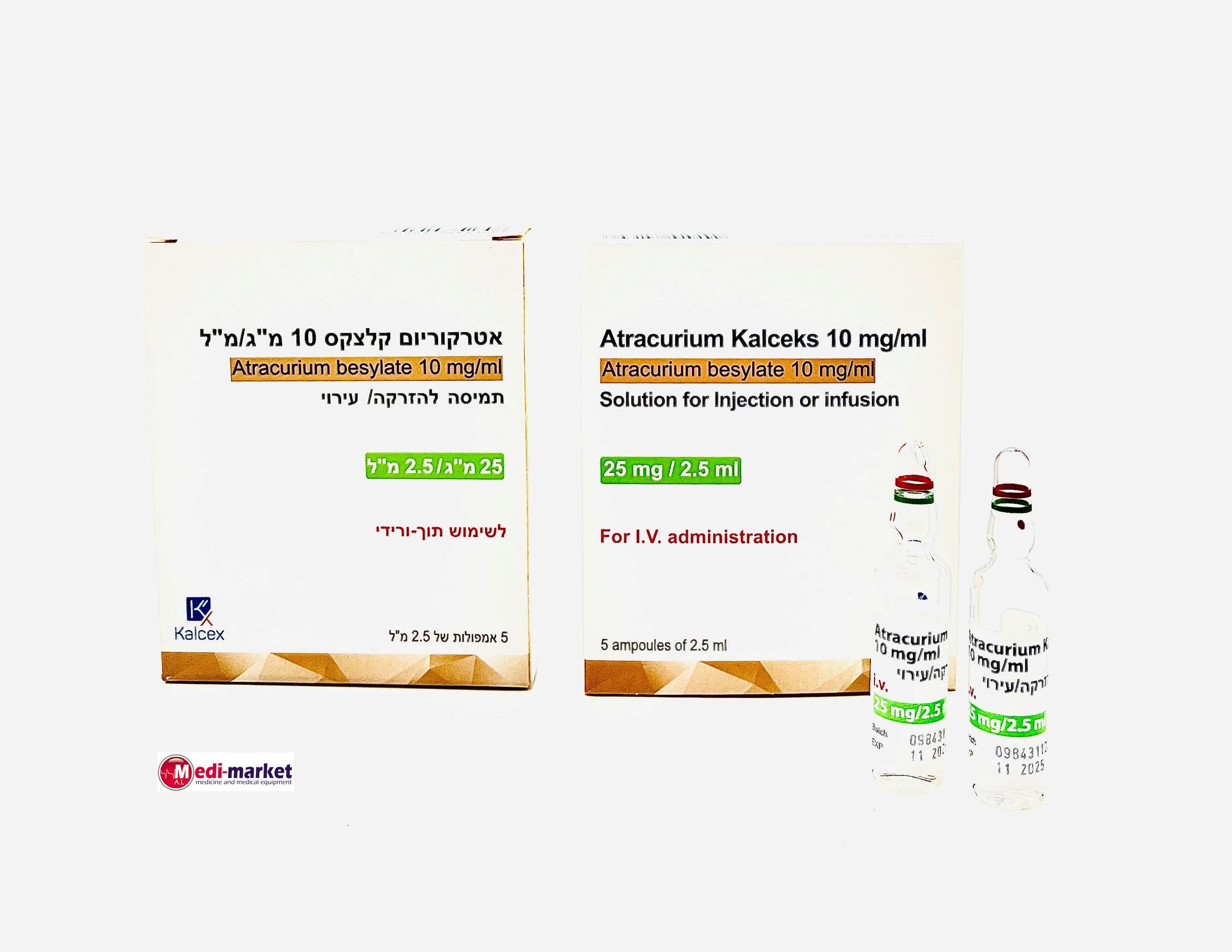
אטרקוריום קלצקס 10 מ"ג/מ"ל ATRACURIUM KALCEKS 10 MG/ML (ATRACURIUM BESYLATE)
תרופה במרשם
תרופה בסל
נרקוטיקה
ציטוטוקסיקה
צורת מתן:
תוך-ורידי : I.V
צורת מינון:
תמיסה להזרקהאינפוזיה : SOLUTION FOR INJECTION / INFUSION
עלון לרופא
מינוניםPosology התוויות
Indications תופעות לוואי
Adverse reactions התוויות נגד
Contraindications אינטראקציות
Interactions מינון יתר
Overdose הריון/הנקה
Pregnancy & Lactation אוכלוסיות מיוחדות
Special populations תכונות פרמקולוגיות
Pharmacological properties מידע רוקחי
Pharmaceutical particulars אזהרת שימוש
Special Warning עלון לרופא
Physicians Leaflet
Special Warning : אזהרת שימוש
4.4 Special warnings and precautions for use Precautions: In common with all the other neuromuscular blocking agents, Atracurium Kalceks paralyses the respiratory muscles as well as other skeletal muscles but has no effect on consciousness. Atracurium Kalceks should be administered only with adequate general anaesthesia and only by or under the close supervision of an experienced anaesthetist with adequate facilities for endotracheal intubation and artificial ventilation. The potential for histamine release exists in susceptible patients during Atracurium Kalceks administration. Caution should be exercised in administering Atracurium Kalceks to patients with a history suggestive of an increased sensitivity to the effects of histamine. In particular, bronchospasm may occur in patients with a history of allergy and asthma. High rates of cross-sensitivity between neuromuscular blocking agents have been reported. Therefore, where possible, before administering atracurium, hypersensitivity to other neuromuscular blocking agents should be excluded. Atracurium should only be used when absolutely essential in susceptible patients. Patients who experience a hypersensitivity reaction under general anaesthesia should be tested subsequently for hypersensitivity to other neuromuscular blockers. Monitoring of serial creatinine phosphate (cpk) values should be considered in asthmatic patients receiving high dose corticosteroids and neuromuscular blocking agents in ICU. Atracurium Kalceks does not have significant vagal or ganglionic blocking properties in the recommended dosage range. Consequently, Atracurium Kalceks has no clinically significant effects on heart rate in the recommended dosage range and it will not counteract the bradycardia produced by many anaesthetic agents or by vagal stimulation during surgery. In common with other non-depolarising neuromuscular blocking agents, increased sensitivity to atracurium may be expected in patients with myasthenia gravis and other forms of neuromuscular disease. As with other neuromuscular blocking agents severe acid-base and/or serum electrolyte abnormalities may increase or decrease the sensitivity of patients to atracurium. As with other non-depolarising neuromuscular blockers hypophosphataemia may prolong recovery. Recovery may be hastened by correcting this condition. Atracurium Kalceks should be administered over a period of 60 seconds to patients who may be unusually sensitive to falls in arterial blood pressure, for example those who are hypovolaemic. Atracurium Kalceks is inactivated by high pH and so must not be mixed in the same syringe with thiopental or any alkaline agent. When a small vein is selected as the injection site, Atracurium Kalceks should be flushed through the vein with physiological saline after injection. When other anaesthetic drugs are administered through the same in-dwelling needle or cannula as Atracurium Kalceks it is important that each drug is flushed through with an adequate volume of physiological saline. Atracurium besilate is hypotonic and must not be administered into the infusion line of a blood transfusion. Studies in malignant hyperthermia in susceptible animals (swine), and clinical studies in patients susceptible to malignant hypothermia indicate that Atracurium Kalceks does not trigger this syndrome. In common with other non-depolarising neuromuscular blocking agents, resistance may develop in patients suffering from burns. Such patients may require increased doses, dependent on the time elapsed since the burn injury and the extent of the burn. Intensive Care Unit (ICU) patients: When administered to laboratory animals in high doses, Laudanosine, a metabolite of atracurium has been associated with transient hypotension and, in some species, cerebral excitatory effects. Although seizures have been seen in ICU patients receiving atracurium, a causal relationship to laudanosine has not been established (see Undesirable Effects).
Effects on Driving
4.7 Effects on ability to drive and use machines This precaution is not relevant to the use of atracurium. Atracurium will always be used in combination with a general anaesthetic and therefore the usual precautions relating to performance of tasks following general anaesthesia apply.

שימוש לפי פנקס קופ''ח כללית 1994
לא צוין
תאריך הכללה מקורי בסל
01/01/1995
הגבלות
תרופה מוגבלת לשימוש בבתי חולים או אשפוז יום
מידע נוסף
