Quest for the right Drug
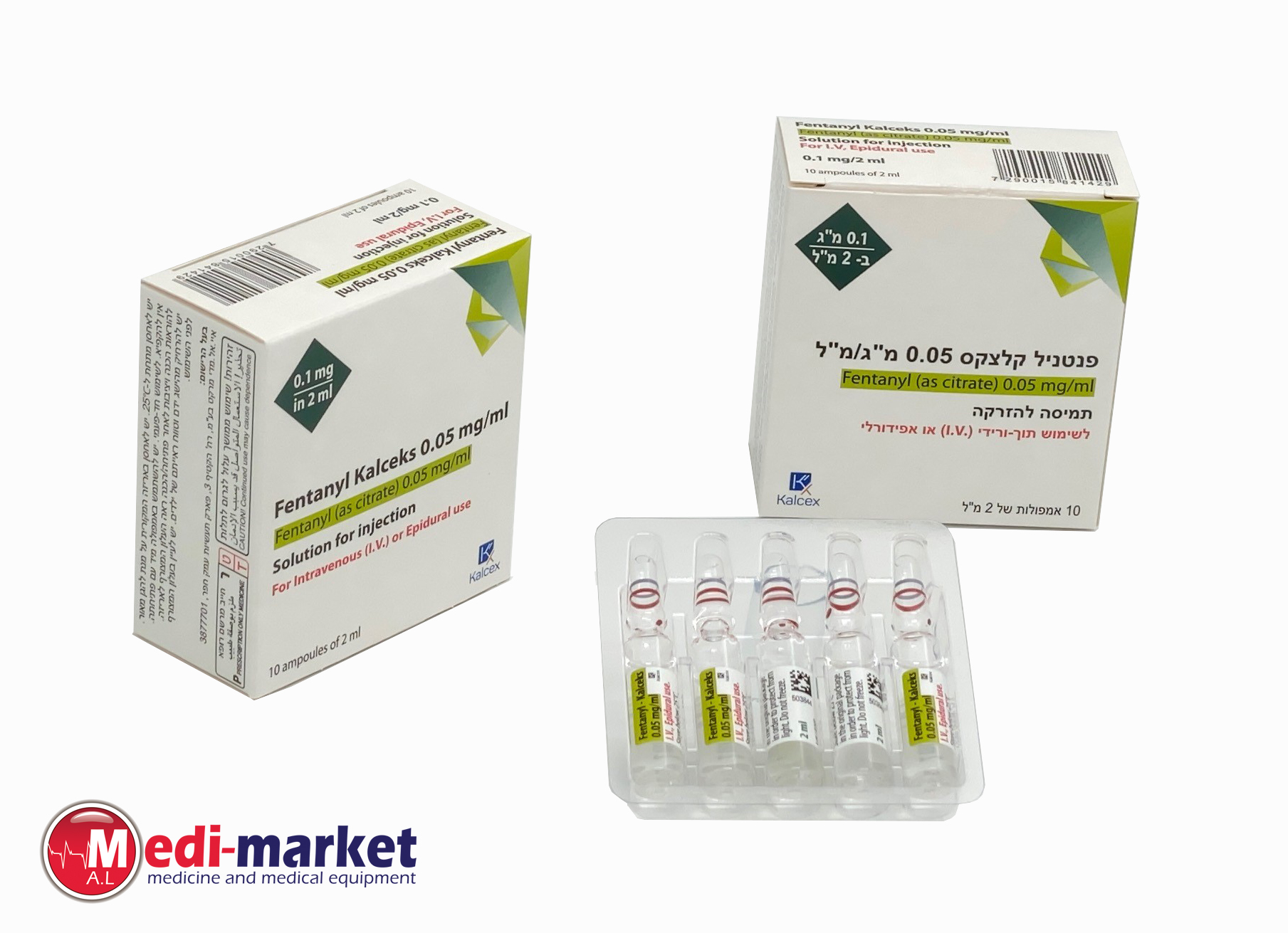
פנטניל - קלצקס 0.05 מ"ג/מ"ל FENTANYL - KALCEKS 0.05 MG/ML (FENTANYL AS CITRATE)
תרופה במרשם
תרופה בסל
נרקוטיקה
ציטוטוקסיקה
צורת מתן:
אפידורל, תוך-ורידי : EPIDURAL, I.V
צורת מינון:
תמיסה להזרקה : SOLUTION FOR INJECTION
עלון לרופא
מינוניםPosology התוויות
Indications תופעות לוואי
Adverse reactions התוויות נגד
Contraindications אינטראקציות
Interactions מינון יתר
Overdose הריון/הנקה
Pregnancy & Lactation אוכלוסיות מיוחדות
Special populations תכונות פרמקולוגיות
Pharmacological properties מידע רוקחי
Pharmaceutical particulars אזהרת שימוש
Special Warning עלון לרופא
Physicians Leaflet
Special Warning : אזהרת שימוש
4.4 Special warnings and precautions for use Warnings: Tolerance and Opioid use disorder (abuse and dependence) Tolerance, physical dependence, and psychological dependence may develop upon repeated administration of opioids. Repeated use of opioids may lead to Opioid use disorder (OUD). Abuse or intentional misuse of opioids may result in overdose and/or death. The risk of developing OUD is increased in patients with a personal or a family history (parents or siblings) of substance use disorders (including alcohol use disorder), in current tobacco users or in patients with a personal history of other mental health disorders (e.g. major depression, anxiety and personality disorders). For all patients, prolonged use of this product may lead to drug dependence (addiction), even at therapeutic doses. The risks are increased in individuals with current or past history of substance misuse disorder (including alcohol misuse) or mental health disorder (e.g., major depression). Additional support and monitoring may be necessary when prescribing for patients at risk of opioid misuse. A comprehensive patient history should be taken to document concomitant medications, including over-the-counter medicines and medicines obtained on-line, and past and present medical and psychiatric conditions. Patients may find that treatment is less effective with chronic use and express a need to increase the dose to obtain the same level of pain control as initially experienced. Patients may also supplement their treatment with additional pain relievers. These could be signs that the patient is developing tolerance. The risks of developing tolerance should be explained to the patient. Overuse or misuse may result in overdose and/or death. It is important that patients only use medicines that are prescribed for them at the dose they have been prescribed and do not give this medicine to anyone else. Patients should be closely monitored for signs of misuse, abuse, or addiction. The clinical need for analgesic treatment should be reviewed regularly. Drug withdrawal syndrome Prior to starting treatment with any opioids, a discussion should be held with patients to put in place a withdrawal strategy for ending treatment with fentanyl. Drug withdrawal syndrome may occur upon abrupt cessation of therapy or dose reduction. When a patient no longer requires therapy, it is advisable to taper the dose gradually to minimise symptoms of withdrawal. Tapering from a high dose may take weeks to months. The opioid drug withdrawal syndrome is characterised by some or all of the following: restlessness, lacrimation, rhinorrhoea, yawning, perspiration, chills, myalgia, mydriasis and palpitations. Other symptoms may also develop including irritability, agitation, anxiety, hyperkinesia, tremor, weakness, insomnia, anorexia, abdominal cramps, nausea, vomiting, diarrhoea, increased blood pressure, increased respiratory rate or heart rate. If women take this drug during pregnancy, there is a risk that their new-born infants will experience neonatal withdrawal syndrome. Hyperalgesia Hyperalgesia may be diagnosed if the patient on long-term opioid therapy presents with increased pain. This might be qualitatively and anatomically distinct from pain related to disease progression or to breakthrough pain resulting from development of opioid tolerance. Pain associated with hyperalgesia tends to be more diffuse than the pre-existing pain and less defined in quality. Symptoms of hyperalgesia may resolve with a reduction of opioid dose. Following intravenous administration of fentanyl, a transient fall in blood pressure may occur, especially in hypovolaemic patients. Appropriate measures to maintain a stable arterial pressure should be taken. Respiratory Depression As with all potent opioids, profound analgesia is accompanied by marked respiratory depression, which may persist into or recur in the early postoperative period. Care should be taken after large doses or infusions of fentanyl to ensure that adequate spontaneous breathing has been established and maintained before discharging the patient from the recovery area. Significant respiratory depression will occur following the administration of fentanyl in doses in excess of 200 mcg. This, and the other pharmacological effects of fentanyl, can be reversed by specific opioid antagonists, but additional doses may be necessary because the respiratory depression may last longer than the duration of action of the opioid antagonist. Resuscitation equipment and opioid antagonists should be readily available. Hyperventilation during anaesthesia may alter the patient’s response to CO2, thus affecting respiration postoperatively. Administration in labour may cause respiratory depression in the new-born infant. Cardiac disease Bradycardia, and possibly cardiac arrest, can occur if the patient has received an insufficient amount of anticholinergic, or when fentanyl is combined with non-vagolytic muscle relaxants. Bradycardia can be antagonised by atropine. Muscle rigidity Muscular rigidity (morphine-like effect) may occur. Rigidity, which may also involve the thoracic muscles, can be avoided by the following measures: − slow IV injection (usually sufficient for lower doses); − premedication with benzodiazepines; − use of muscle relaxants. Non-epileptic (myo)clonic movements can occur. Precautions: Fentanyl should be given only in an environment where the airway can be controlled and by personnel who can control the airway. Special dosing conditions The use of rapid bolus injections of opioids should be avoided in patients with compromised intracerebral compliance; in such patients the transient decrease in the mean arterial pressure has occasionally been accompanied by a transient reduction of the cerebral perfusion pressure. It is recommended to reduce dosage in the elderly and debilitated patients. In uncontrolled hypothyroidism, pulmonary disease, decreased respiratory reserve, alcoholism and hepatic or renal impairment the dosage should be titrated with care and prolonged post- operative monitoring is required. Patients on chronic opioid therapy or with a history of opioid abuse may require higher doses. Myasthenia gravis In patients with myasthenia gravis, careful consideration should be applied in the use of certain anticholinergic agents and neuromuscular-blocking pharmaceutical agents prior to, and during, the administration of a general anaesthetic regimen which includes administering intravenous fentanyl. Interaction with neuroleptics If fentanyl is administered with a neuroleptic, the user should be familiar with the special properties of each drug, particularly the difference in duration of action. When such a combination is used, there is a higher incidence of hypotension. Neuroleptics can induce extrapyramidal symptoms that can be controlled with anti-Parkinson agents. Bile duct As with other opioids, due to the anticholinergic effects, administration of fentanyl may lead to increases of bile duct pressure and, in isolated cases, spasms of the Sphincter of Oddi might be observed. Serotonin Syndrome Caution is advised when fentanyl is co-administered with drugs that affect the serotonergic neurotransmitter systems. The development of a potentially life-threatening serotonin syndrome may occur with the concomitant use of serotonergic drugs such as Selective Serotonin Re-uptake Inhibitors (SSRIs) and Serotonin Norepinephrine Re-uptake Inhibitors (SNRIs), and with drugs which impair metabolism of serotonin (including Monoamine Oxidase Inhibitors [MAOIs]). This may occur within the recommended dose. Serotonin syndrome may include mental-status changes (e.g., agitation, hallucinations, coma), autonomic instability (e.g., tachycardia, labile blood pressure, hyperthermia), neuromuscular abnormalities (e.g., hyperoreflexia, incoordination, rigidity), and/or gastrointestinal symptoms (e.g., nausea, vomiting, diarrhoea). If serotonin syndrome is suspected, rapid discontinuation of fentanyl should be considered. Risk from concomitant use of Central Nervous System (CNS) depressants, especially benzodiazepines or related drugs Concomitant use of fentanyl and CNS depressants especially benzodiazepines or related drugs in spontaneous breathing patients, may increase the risk of profound sedation, respiratory depression, coma and death. If a decision is made to administer fentanyl concomitantly with a CNS depressant, especially a benzodiazepine or a related drug, the lowest effective dose of both drugs should be administered, for the shortest period of concomitant use. Patients should be carefully monitored for signs and symptoms of respiratory depression and profound sedation. In this respect, it is strongly recommended to inform patients and their caregivers to be aware of these symptoms (see Interactions). Paediatric population Techniques that involve analgesia in a spontaneously breathing child should only be used as part of an anaesthetic technique, or given as part of a sedation / analgesia technique, with experienced personnel in an environment that can manage sudden chest wall rigidity requiring intubation, or apnoea requiring airway support. This medicinal product contains: 7.08 mg sodium per 2 ml ampoule, that is to say essentially ‘sodium-free’. 35.41 mg sodium per 10 ml ampoule, equivalent to 1.78% of the WHO recommended maximum daily intake of 2 g sodium for an adult. To be taken into consideration by patients on a controlled sodium diet.
Effects on Driving
4.7 Effects on ability to drive and use machines Where early discharge is envisaged, patients should be advised not to drive or operate machinery for at least 24 hours following administration. This medicine can impair cognitive function and can affect a patient’s ability to drive safely. When prescribing this medicine, patients should be told: • The medicine is likely to affect your ability to drive • Do not drive until you know how the medicine affects you

שימוש לפי פנקס קופ''ח כללית 1994
לא צוין
תאריך הכללה מקורי בסל
לא צוין
הגבלות
לא צוין
מידע נוסף
