Quest for the right Drug
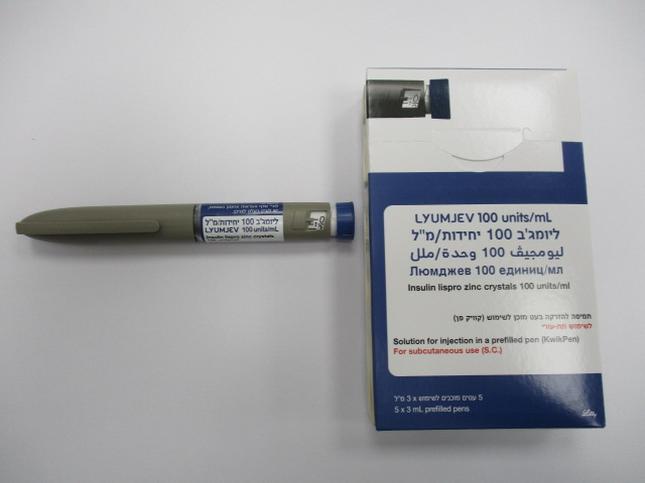
ליומג'ב 100 יחידות/מ"ל LYUMJEV 100 UNITS/ML (INSULIN LISPRO, INSULIN LISPRO AS ZINC CRYSTALS)
תרופה במרשם
תרופה בסל
נרקוטיקה
ציטוטוקסיקה
צורת מתן:
תת-עורי, תוך-ורידי : S.C, I.V
צורת מינון:
תמיסה להזרקה : SOLUTION FOR INJECTION
עלון לרופא
מינוניםPosology התוויות
Indications תופעות לוואי
Adverse reactions התוויות נגד
Contraindications אינטראקציות
Interactions מינון יתר
Overdose הריון/הנקה
Pregnancy & Lactation אוכלוסיות מיוחדות
Special populations תכונות פרמקולוגיות
Pharmacological properties מידע רוקחי
Pharmaceutical particulars אזהרת שימוש
Special Warning עלון לרופא
Physicians Leaflet
Posology : מינונים
4.2 Posology and method of administration Posology Lyumjev is a mealtime insulin for subcutaneous injection and should be administered zero to two minutes before the start of the meal (see section 5.1). Due to its rapid onset of activity, Lyumjev can be administered up to 20 minutes after starting a meal. To achieve the most optimal glycemic control, the bolus injection after the start of the meal should be given only in exceptional cases. Lyumjev 100 units/mL is suitable for continuous subcutaneous insulin infusion (CSII) and is used for both the bolus and basal insulin requirement. The initial dose should take into account the type of diabetes, weight of the patient and their blood glucose levels. The early onset of action must be considered when prescribing Lyumjev (see section 5.1). Continued adjustment of the dose of Lyumjev should be based on the patient’s metabolic needs, blood glucose monitoring results, and glycaemic control goal. Dose adjustments may be needed, when switching from another insulin, with changes in physical activity, changes in concomitant medicinal products, changes in meal patterns (i.e., amount and type of food, timing of food intake), changes in renal or hepatic function or during acute illness to minimize the risk of hypoglycaemia or hyperglycaemia (see sections 4.4 and 4.5). Switching from another mealtime insulin medicinal product If converting from another mealtime insulin to Lyumjev, the change can be done on a unit-to-unit basis. The potency of insulin analogues, including Lyumjev, is expressed in units. One (1) unit of Lyumjev corresponds to 1 international unit (IU) of human insulin or 1 unit of other fast-acting insulin analogues. Missed doses Patients who forget a mealtime dose should monitor their blood glucose level to decide if an insulin dose is needed, and to resume their usual dosing schedule at the next meal. Special populations Elderly (≥ 65 years old) The safety and efficacy of Lyumjev has been established in elderly patients aged 65 to 75 years. Close glucose monitoring is recommended and the insulin dose should be adjusted on an individual basis (see sections 4.8, 5.1 and 5.2). The therapeutic experience in patients ≥ 75 years of age is limited. Renal impairment Insulin requirements may be reduced in the presence of renal impairment. In patients with renal impairment, glucose monitoring should be intensified and the dose adjusted on an individual basis. Hepatic impairment Insulin requirements may be reduced in patients with hepatic impairment due to reduced capacity for gluconeogenesis and reduced insulin breakdown. In patients with hepatic impairment, glucose monitoring should be intensified and the dose adjusted on an individual basis. Paediatric population Lyumjev can be used safely and effectively in adolescents and children (see section 5.1). Lyumjev is recommended to be administered zero to two minutes before the start of the meal. Method of administration Patients should be trained on proper use and injection technique before initiating Lyumjev. Patients should be told to: • Always check insulin labels before administration. • Inspect Lyumjev visually before use and discard for particulate matter or discolouration. • Injection or infusion sites should always be rotated within the same region in order to reduce the risk of lipodystrophy and cutaneous amyloidosis (see section 4.4 and 4.8). • Carry a spare or alternative administration method in case their delivery system breaks. Subcutaneous injection Lyumjev should be injected subcutaneously into the abdomen, upper arm, thigh or buttocks (see section 5.2). Lyumjev should generally be used in combination with an intermediate or long-acting insulin. A different injection site should be used if injecting at the same time as another insulin. When injecting a blood vessel should not be entered. Devices should be discarded if any part looks broken or damaged. The needle should be discarded after each injection. Lyumjev vials If subcutaneous administration by syringe is necessary, a vial should be used. The appropriate syringe must have 100 unit markings. Patients using vials must never share needles or syringes. Lyumjev cartridges Lyumjev in cartridges is only suitable for subcutaneous injections from a Lilly reusable pen. Lyumjev cartridges should not be used with any other reusable pen as the dosing accuracy has not been established with other pens. The instructions with each individual pen must be followed for loading the cartridge, attaching the needle and administering the insulin injection. To prevent the possible transmission of disease, each cartridge must be used by one patient only, even if the needle on the delivery device is changed. Lyumjev KwikPens The KwikPen and Junior KwikPen are only suitable for subcutaneous injections. Lyumjev KwikPens are available in two concentrations: Lyumjev 100 units/mL KwikPen and Lyumjev 200 units/mL KwikPen. See the separate SmPC for Lyumjev 200 units/mL KwikPen. The KwikPen delivers 1 - 60 units in steps of 1 unit in a single injection. The Lyumjev 100 units/mL Junior KwikPen delivers 0.5 - 30 units in steps of 0.5 units in a single injection. The number of insulin units is shown in the dose window of the pen regardless of concentration and no dose conversion should be done when transferring a patient to a new concentration or to a pen with a different dose step. Lyumjev 100 units/mL Junior KwikPen is suitable for patients who may benefit from finer insulin dose adjustments. For detailed user instructions, please refer to the instructions for use provided with the package leaflet. To prevent the possible transmission of disease, each pen must be used by one patient only, even if the needle is changed. CSII (insulin pump) Use a pump suitable for insulin infusion. Fill the pump reservoir from a Lyumjev 100 units/mL vial. Patients using a pump should follow the instructions provided with the pump and infusion set. Use the correct reservoir and catheter for the pump. When filling the pump reservoir avoid damaging it by using the correct needle length on the filling system. The infusion set (tubing and cannula) should be changed in accordance with the instructions in the product information supplied with the infusion set. A pump malfunction or obstruction of the infusion set can result in a rapid rise in glucose levels (see section 4.4). Intravenous use Lyumjev 100 units/mL is available in vials if administration of intravenous injection is necessary. This medicinal product must not be mixed with any other insulin or any other medicinal product except those mentioned in section 6.6. For instructions on dilution of the medicinal product before administration, see section 6.6. Intravenous administration of Lyumjev 100 units/mL must be performed under medical supervision.

פרטי מסגרת הכללה בסל
התרופה האמורה תינתן לטיפול בחולי סוכרת.
מסגרת הכללה בסל
התוויות הכלולות במסגרת הסל
| התוויה | תאריך הכללה | תחום קליני | Class Effect | מצב מחלה |
|---|---|---|---|---|
| התרופה האמורה תינתן לטיפול בחולי סוכרת. | 09/03/1999 | מחלות מטבוליות | INSULIN ASPART, INSULIN GLULISINE, INSULIN LISPRO |
שימוש לפי פנקס קופ''ח כללית 1994
לא צוין
תאריך הכללה מקורי בסל
09/03/1999
הגבלות
תרופה מוגבלת לרישום ע'י רופא מומחה או הגבלה אחרת
מידע נוסף
עלון מידע לצרכן
19.04.22 - עלון לצרכן עברית 24.11.22 - עלון לצרכן אנגלית 24.11.22 - עלון לצרכן אנגלית 24.11.22 - עלון לצרכן עברית 24.11.22 - עלון לצרכן עברית 24.11.22 - עלון לצרכן ערבית 24.11.22 - עלון לצרכן ערבית 28.12.23 - עלון לצרכן עברית 28.12.23 - עלון לצרכן עברית 01.01.24 - עלון לצרכן עברית 18.03.24 - עלון לצרכן אנגלית 21.03.24 - עלון לצרכן אנגלית 18.03.24 - עלון לצרכן ערבית 21.03.24 - עלון לצרכן ערבית 28.06.24 - עלון לצרכן עברית 28.06.24 - עלון לצרכן עברית 28.06.24 - עלון לצרכן עברית 11.07.24 - עלון לצרכן אנגלית 11.07.24 - עלון לצרכן ערבית 28.09.24 - עלון לצרכן אנגלית 28.09.24 - עלון לצרכן אנגלית 28.09.24 - עלון לצרכן ערבית 28.09.24 - עלון לצרכן ערביתלתרופה במאגר משרד הבריאות
ליומג'ב 100 יחידות/מ"ל