Quest for the right Drug
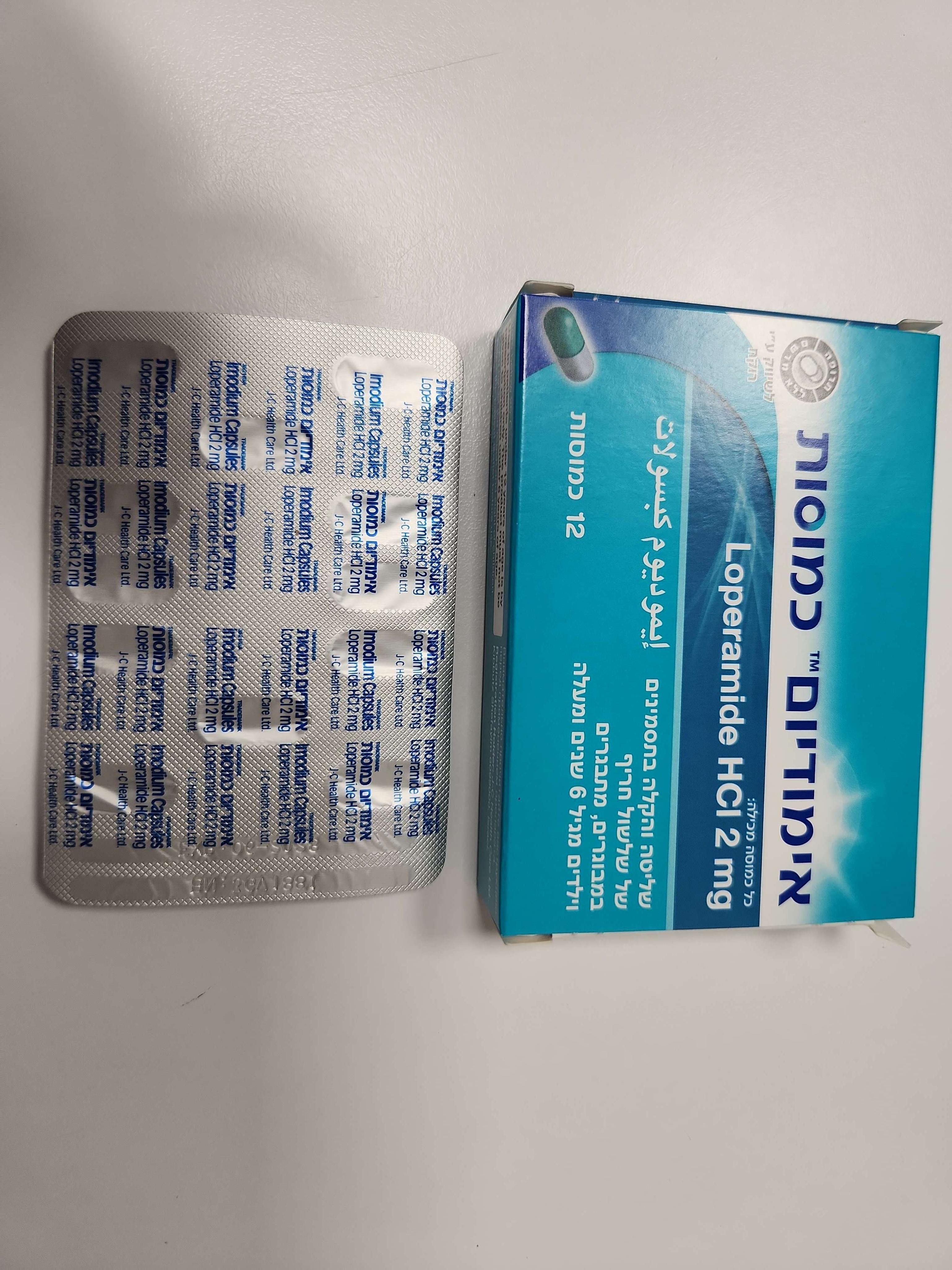
אימודיום כמוסות IMODIUM CAPSULES (LOPERAMIDE HYDROCHLORIDE)
תרופה במרשם
תרופה בסל
נרקוטיקה
ציטוטוקסיקה
צורת מתן:
פומי : PER OS
צורת מינון:
קפסולות : CAPSULES
עלון לרופא
מינוניםPosology התוויות
Indications תופעות לוואי
Adverse reactions התוויות נגד
Contraindications אינטראקציות
Interactions מינון יתר
Overdose הריון/הנקה
Pregnancy & Lactation אוכלוסיות מיוחדות
Special populations תכונות פרמקולוגיות
Pharmacological properties מידע רוקחי
Pharmaceutical particulars אזהרת שימוש
Special Warning עלון לרופא
Physicians Leaflet
Special Warning : אזהרת שימוש
4.4 Special warnings and precautions for use The treatment of diarrhea with loperamide HCl is only symptomatic. Whenever an underlying etiology can be determined, specific treatment should be given when appropriate. The priority in acute diarrhea is the prevention or reversal of fluid and electrolyte depletion. This is particularly important in young children and in frail and elderly patients with acute diarrhea. Use of this medicine does not preclude the administration of appropriate fluid and electrolyte replacement therapy. Since persistent diarrhea can be an indicator of potentially more serious conditions, this medicine should not be used for prolonged periods until the underlying cause of the diarrhea has been investigated. In acute diarrhea, if clinical improvement is not observed within 48 hours, the administration of Imodium should be discontinued and patients should be advised to consult their doctor. Patients with AIDS treated with this medicine for diarrhea should have therapy stopped at the earliest signs of abdominal distension. There have been isolated reports of obstipation with an increased risk for toxic megacolon in AIDS patients with infectious colitis from both viral and bacterial pathogens treated with loperamide hydrochloride. Although no pharmacokinetic data are available in patients with hepatic impairment, this medicine should be used with caution in such patients because of reduced first pass metabolism, as it may result in a relative overdose leading to CNS toxicity. Patients with rare hereditary problems of galactose intolerance, the Lapp lactase deficiency or glucose- galactose malabsorption should not take this medicine because it contains lactose. Pediatric population In children aged between 6 and 12 years, IMODIUM should only be used under medical supervision. The data available regarding the use of loperamide HCl in children under the age of 12 years are limited (see section 4.8 "Undesirable effects"). Cardiac events including QT interval and QRS complex prolongation and torsades de pointes have been reported in association with overdose. Some cases had a fatal outcome (see section 4.9). Overdose can unmask existing Brugada syndrome. Patients should not exceed the recommended dose and/or the recommended duration of treatment. Caution is needed in patients with a history of drug abuse. Abuse and misuse of loperamide, has been described (see section 4.9). Loperamide is an opioid with low bioavailability and limited potential to penetrate the blood brain barrier at therapeutic doses. However, addiction is observed with opioids as a class.
Effects on Driving
4.7 Effects on ability to drive and use machines Loss of consciousness, depressed level of consciousness, tiredness, dizziness, or drowsiness may occur when diarrhea is treated with this medicine. Therefore, it is advisable to exercise caution when driving a car or operating machinery. See Section 4.8, Undesirable Effects.

שימוש לפי פנקס קופ''ח כללית 1994
Diarrhea
תאריך הכללה מקורי בסל
01/01/1995
הגבלות
תרופה שאושרה לשימוש כללי בקופ'ח
מידע נוסף
עלון מידע לצרכן
26.04.18 - עלון לצרכן 31.08.22 - עלון לצרכן אנגלית 31.08.22 - עלון לצרכן עברית 31.08.22 - עלון לצרכן ערבית 17.11.22 - עלון לצרכן אנגלית 17.11.22 - עלון לצרכן עברית 17.11.22 - עלון לצרכן ערבית 16.03.23 - עלון לצרכן אנגלית 16.03.23 - עלון לצרכן עברית 16.03.23 - עלון לצרכן ערבית 14.08.23 - עלון לצרכן אנגלית 14.08.23 - עלון לצרכן עברית 14.08.23 - עלון לצרכן ערבית 25.05.16 - החמרה לעלון 02.10.18 - החמרה לעלון 03.06.20 - החמרה לעלון 31.08.22 - החמרה לעלון 17.11.22 - החמרה לעלון 16.03.23 - החמרה לעלוןלתרופה במאגר משרד הבריאות
אימודיום כמוסות