Quest for the right Drug
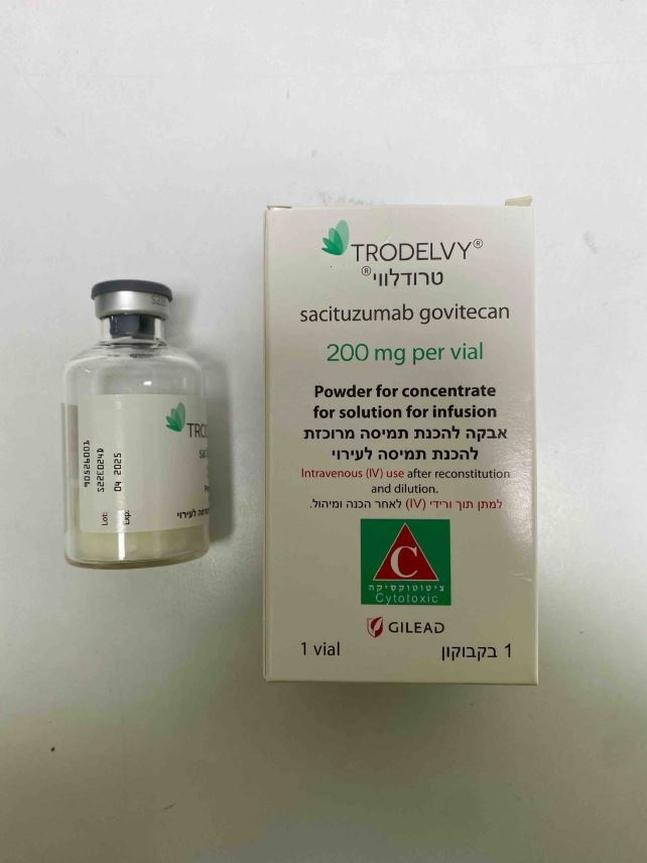
טרודלווי TRODELVY (SACITUZUMAB GOVITECAN)
תרופה במרשם
תרופה בסל
נרקוטיקה
ציטוטוקסיקה
צורת מתן:
תוך-ורידי : I.V
צורת מינון:
אין פרטים : POWDER FOR CONCENTRATE FOR SOLUTION FOR INFUSION
עלון לרופא
מינוניםPosology התוויות
Indications תופעות לוואי
Adverse reactions התוויות נגד
Contraindications אינטראקציות
Interactions מינון יתר
Overdose הריון/הנקה
Pregnancy & Lactation אוכלוסיות מיוחדות
Special populations תכונות פרמקולוגיות
Pharmacological properties מידע רוקחי
Pharmaceutical particulars אזהרת שימוש
Special Warning עלון לרופא
Physicians Leaflet
Adverse reactions : תופעות לוואי
4.8 Undesirable effects Summary of the safety profile The most common adverse reactions reported in patients treated with sacituzumab govitecan were: neutropenia (67.6%), nausea (62.6%), diarrhoea (62.5%), fatigue (61.5%), alopecia (45.6%), anaemia (40.7%), constipation (36.2%), vomiting (33.6%), decreased appetite (25.7%), dyspnoea (22.1%) and abdominal pain (20.2%). The most common grade 3 or higher adverse reactions were neutropenia (50.7%), leukopenia (10.5%), diarrhoea (10.3%), anaemia (9.3%), fatigue (6.8%), febrile neutropenia (6.1%), hypophosphataemia (4.2%), dyspnoea (3.1%), lymphopenia (2.9%), abdominal pain (2.8%), nausea (2.8%), vomiting (2.5%), hypokalaemia (2.5%), pneumonia (2.3%) and aspartate aminotransferase increased (2.2%). The most frequently reported serious adverse reactions in patients treated with sacituzumab govitecan were febrile neutropenia (4.8%), diarrhoea (3.9%), neutropenia (2.6%) and pneumonia (2%). Tabulated list of adverse reactions The frequencies of adverse reactions are based on pooled data from three clinical studies involving 688 patients who received sacituzumab govitecan 10 mg/kg body weight for the treatment of metastatic TNBC and HR+/HER2- breast cancer. The median exposure to sacituzumab govitecan in this data set was 4.63 months. The adverse reaction frequencies are based on all-cause adverse event frequencies, where a proportion of the events for an adverse reaction may have other causes than sacituzumab govitecan, such as the disease, other medicinal products or unrelated causes. The severity of adverse drug reactions was assessed based on the Common Terminology Criteria for Adverse Events (CTCAE), defining grade 1 = mild, grade 2 = moderate, grade 3 = severe, grade 4 = life threatening, and 5 = death. Adverse reactions are listed by System Organ Class and frequency category. Frequency categories are defined as: very common (≥ 1/10); common (≥ 1/100 to < 1/10); uncommon (≥ 1/1 000 to < 1/100); rare (≥ 1/10 000 to < 1/1 000); very rare (< 1/10 000); and not known (cannot be estimated from the available data). Within each frequency grouping, adverse reactions are presented in the order of decreasing seriousness. Table 2: List of adverse reactions System organ class (SOC) Frequency Adverse reactions Infections and infestations Very common Urinary tract infection Upper respiratory tract infection Common Sepsis Pneumonia Influenza Bronchitis Nasopharyngitis Sinusitis Oral herpes Blood and lymphatic system disorders Very common Neutropenia1 Anaemia2 Leukopenia3 Lymphopenia4 Common Febrile neutropenia Thrombocytopenia5 Immune system disorders Very common Hypersensitivity6 Metabolism and nutrition disorders Very common Decreased appetite Hypokalaemia Hypomagnesaemia Common Dehydration Hyperglycaemia Hypophosphataemia Hypocalcaemia Hyponatraemia Psychiatric disorders Very common Insomnia Common Anxiety Nervous system disorders Very common Headache Dizziness Common Dysgeusia Vascular disorders Common Hypotension Respiratory, thoracic and mediastinal disorders Very common Dyspnoea7 Cough Common Epistaxis Productive cough Rhinorrhoea Nasal congestion Upper airway cough syndrome Gastrointestinal disorders Very common Diarrhoea Vomiting Nausea Constipation Abdominal Pain Common Neutropenic colitis8 Colitis Stomatitis Abdominal pain upper Dyspepsia Gastrooesophageal reflux disease Abdominal distension Uncommon Enteritis Skin and subcutaneous tissue disorders Very common Alopecia Rash Pruritus Common Rash maculopapular Skin hyperpigmentation Dermatitis acneiform Dry skin Musculoskeletal and connective tissue disorders Very common Back pain Arthralgia Common Musculoskeletal chest pain Muscle spasms Renal and urinary disorders Common Haematuria Proteinuria Dysuria General disorders and administration site conditions Very common Fatigue9 Common Pain Chills Investigations Common Weight decreased Blood alkaline phosphatase increased Activated partial thromboplastin time prolonged Blood lactate dehydrogenase increased Injury, poisoning and procedural complications Uncommon Infusion related reaction 1: Includes the following preferred terms: neutropenia; neutrophil count decreased. 2: Includes the following preferred terms: anaemia; haemoglobin decreased; red blood cell count decreased. 3: Includes the following preferred terms: leukopenia; white blood cell count decreased. 4: Includes the following preferred terms: lymphopenia; lymphocyte count decreased. 5: Includes the following preferred terms: thrombocytopenia; platelet count decreased. 6: Hypersensitivity events reported up to the end of the day after treatment was administered. Includes events coded to the following preferred terms: dyspnoea; hypotension; flushing; erythema; chest discomfort; rhinitis allergic; wheezing; oedema; urticaria; anaphylactic reaction; mouth ulceration; skin exfoliation; swollen tongue; throat tightness. 7: Includes the following preferred terms: dyspnoea; dyspnoea exertional 8: Includes the preferred term of neutropenic colitis and events reported as typhlitis 9: Includes the following preferred terms: fatigue, asthenia Description of selected adverse reactions Neutropenia The median time to onset of neutropenia (including febrile neutropenia) following the start of the first treatment cycle was 16 days. The median duration of neutropenia was 8 days. Neutropenia occurred in 67.6% (465/688) of patients treated with sacituzumab govitecan, including Grade 3-4 neutropenia in 50.7% of patients. Neutropenia was the reason for dose reduction in 12.4% of patients. Neutropenic colitis was observed in 1% (7/688) of patients. Febrile neutropenia occurred in 6.1% (42/688) of patients treated with sacituzumab govitecan. Febrile neutropenia was the reason for dose reduction in 2.9% of patients. Use in patients with reduced UGT1A1 activity The incidence of Grade 3-4 neutropenia was 60.6% (43/71) in patients homozygous for the UGT1A1*28 allele, 52.9% (144/272) in patients heterozygous for the UGT1A1*28 allele, and 49.1% (140/285) in patients homozygous for the wild-type allele. The incidence of Grade 3-4 febrile neutropenia was 14.1% (10/71) in patients homozygous for the UGT1A1*28 allele, 5.9% (16/272) in patients heterozygous for the UGT1A1*28 allele, and 4.6% (13/285) in patients homozygous for the wild-type allele. The incidence of Grade 3-4 anaemia was 15.5% (11/71) in patients homozygous for the UGT1A1*28 allele, 7.4% (20/272) in patients heterozygous for the UGT1A1*28 allele, and 8.1% (23/285) in patients homozygous for the wild-type allele. Compared to patients homozygous for the wild-type allele, earlier median onset of neutropenia and anaemia was observed in patients homozygous for the UGT1A1*28 allele and in patients heterozygous for the UGT1A1*28 allele. Diarrhoea The median time to onset of diarrhoea following the start of the first treatment cycle was 13 days. The median duration of diarrhoea was 8 days. Diarrhoea occurred in 62.5% (430/688) of patients treated with sacituzumab govitecan. Grade 3 events occurred in 10.3% (71/688) of patients. Three of 688 patients (< 1%) discontinued treatment because of diarrhoea. Hypersensitivity Hypersensitivity reactions reported up to the end of the day following dosing occurred in 33.0% (227/688) of patients treated with sacituzumab govitecan. Grade 3 and above hypersensitivity occurred in 1.7% (12/688) of patients treated with sacituzumab govitecan. The incidence of hypersensitivity reactions leading to permanent discontinuation of sacituzumab govitecan was 0.1% (1/688). Immunogenicity Across clinical studies in patients treated with sacituzumab govitecan, 9 (1.1%) of 785 patients developed antibodies to sacituzumab govitecan; 6 of these patients (0.8% of all patients treated with sacituzumab govitecan) had neutralizing antibodies against sacituzumab govitecan. Special Populations There was no difference in discontinuation rate due to adverse events in patients aged 65 years or older compared with younger patients with mTNBC. There was a higher discontinuation rate due to adverse reactions in patients aged 65 years or older (14%) compared with younger patients (3%) with HR+/HER2- metastatic breast cancer. There was a higher incidence rate of serious adverse events in patients aged 75 years or older (67%) compared to patients aged 65 years or older (43%) and patients younger than 65 years (24%) with HR+/HER2- metastatic breast cancer. Reporting of suspected adverse reactions Reporting suspected adverse reactions after authorisation of the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the medicinal product. You can report any side effects to the Ministry of Health by clicking on the link "Report side effects due to medical treatment" that is located on the Ministry of Health homepage (www.health.gov.il) which redirects to the online form for reporting side effects, or by clicking on the link: https://sideeffects.health.gov.il

פרטי מסגרת הכללה בסל
א. התרופה תינתן כמונותרפיה לטיפול בסרטן שד לא נתיח או גרורתי, מסוג TNBC (Triple negative breast cancer), שקיבלו שני טיפולים סיסטמיים קודמים, שלפחות אחד מהם ניתן למחלה בשלב מתקדם. ב. מתן התרופה האמורה ייעשה לפי מרשם של רופא מומחה באונקולוגיה.
מסגרת הכללה בסל
התוויות הכלולות במסגרת הסל
| התוויה | תאריך הכללה | תחום קליני | Class Effect | מצב מחלה |
|---|---|---|---|---|
| א. התרופה תינתן כמונותרפיה לטיפול בסרטן שד לא נתיח או גרורתי, מסוג TNBC (Triple negative breast cancer), שקיבלו שני טיפולים סיסטמיים קודמים, שלפחות אחד מהם ניתן למחלה בשלב מתקדם. ב. מתן התרופה האמורה ייעשה לפי מרשם של רופא מומחה באונקולוגיה. | 17/03/2024 | אונקולוגיה | סרטן שד מסוג Triple negative, TNBC |
שימוש לפי פנקס קופ''ח כללית 1994
לא צוין
תאריך הכללה מקורי בסל
17/03/2024
הגבלות
תרופה מוגבלת לרישום ע'י רופא מומחה או הגבלה אחרת
ATC
מידע נוסף
