Quest for the right Drug
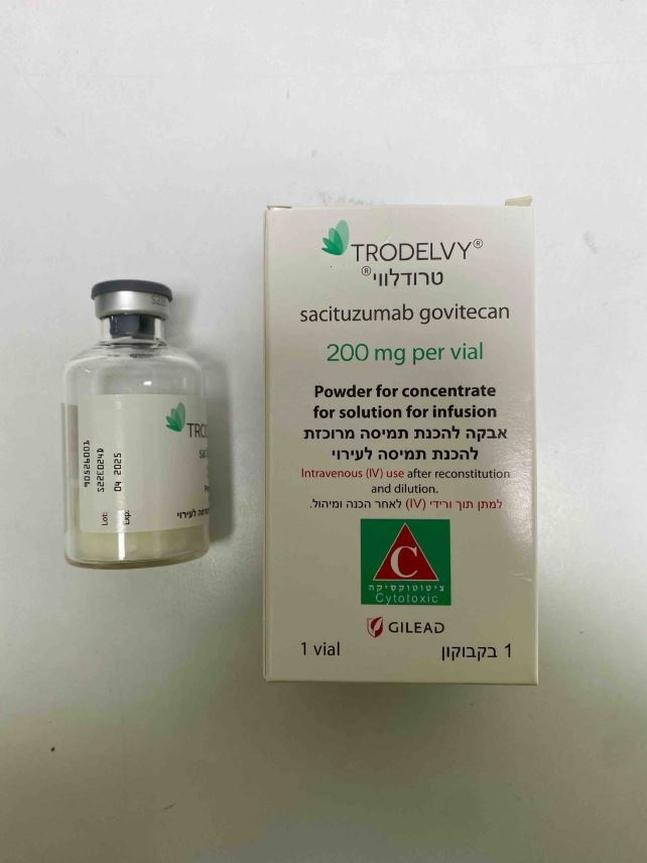
טרודלווי TRODELVY (SACITUZUMAB GOVITECAN)
תרופה במרשם
תרופה בסל
נרקוטיקה
ציטוטוקסיקה
צורת מתן:
תוך-ורידי : I.V
צורת מינון:
אין פרטים : POWDER FOR CONCENTRATE FOR SOLUTION FOR INFUSION
עלון לרופא
מינוניםPosology התוויות
Indications תופעות לוואי
Adverse reactions התוויות נגד
Contraindications אינטראקציות
Interactions מינון יתר
Overdose הריון/הנקה
Pregnancy & Lactation אוכלוסיות מיוחדות
Special populations תכונות פרמקולוגיות
Pharmacological properties מידע רוקחי
Pharmaceutical particulars אזהרת שימוש
Special Warning עלון לרופא
Physicians Leaflet
Pharmaceutical particulars : מידע רוקחי
6. PHARMACEUTICAL PARTICULARS 6.1 List of excipients 2-(N-morpholino)ethane sulfonic acid (MES) Polysorbate 80 (E433) Trehalose dihydrate 6.2 Incompatibilities This medicinal product must not be mixed with other medicinal products except those mentioned in section 6.6. 6.3 Shelf life Unopened vial The expiry date of the product is indicated on the packaging materials. After reconstitution The reconstituted solution should be used immediately to prepare the diluted solution for infusion. If not used immediately, the infusion bag containing diluted solution can be stored in a refrigerator (2°C to 8°C) for up to 24 hours protected from light. 6.4 Special precautions for storage Store in a refrigerator (2°C - 8°C). Do not freeze. Keep the vial in the outer carton in order to protect from light. For storage conditions after reconstitution and dilution of the medicinal product, see section 6.3. 6.5 Nature and contents of container Type I colourless, clear glass 50 mL vial, with an elastomeric butyl stopper and sealed with an aluminum flip-off overseal containing 200 mg of sacituzumab govitecan. Each pack contains one vial. 6.6 Special precautions for disposal and other handling Trodelvy is a cytotoxic medicinal product. Applicable special handling and disposal procedures have to be followed. Reconstitution • Calculate the required dose (mg) of Trodelvy based on the patient’s body weight at the beginning of each treatment cycle (or more frequently if the patient’s body weight changed by more than 10% since the previous administration). • Allow the required number of vials to warm to room temperature (20°C to 25°C). • Using a sterile syringe, slowly inject 20 mL of sodium chloride 9 mg/mL (0.9%) solution for injection into each vial. The resulting concentration will be 10 mg/mL. • Gently swirl vials and allow to dissolve for up to 15 minutes. Do not shake. The product should be inspected visually for particulate matter and discoloration prior to administration. The solution should be free of visible particulates, clear and yellow. Do not use the reconstituted solution if it is cloudy or discoloured. • Use immediately to prepare a diluted solution for infusion. Dilution • Calculate the required volume of the reconstituted solution needed to obtain the appropriate dose according to the patient’s body weight. • Determine the final volume of the infusion solution to deliver the appropriate dose at a sacituzumab govitecan concentration range of 1.1 mg/mL to 3.4 mg/mL. • Withdraw and discard a volume of sodium chloride 9 mg/mL (0.9%) solution for injection from the final infusion bag that is equivalent to the required volume of the reconstituted solution. • Withdraw the calculated amount of the reconstituted solution from the vial(s) using a syringe. Discard any unused portion remaining in the vial(s). • To minimize foaming, slowly inject the required volume of reconstituted solution into a polyvinyl chloride, polyolefin (polypropylene and/or polyethylene) or ethylene vinyl acetate infusion bag. Do not shake the contents. • If necessary, adjust the volume in the infusion bag as needed with sodium chloride 9 mg/mL (0.9%) solution for injection, to obtain a concentration of 1.1 mg/mL to 3.4 mg/mL. Only sodium chloride 9 mg/mL (0.9%) solution for injection should be used since the stability of the reconstituted product has not been determined with other infusion-based solutions. • If not used immediately, the infusion bag containing diluted solution can be stored refrigerated 2°C to 8°C for up to 24 hours protected from light. Do not freeze. After refrigeration, administer the diluted solution at room temperature up to 25°C within 8 hours (including infusion time). Administration • Administer Trodelvy as an intravenous infusion. Protect the infusion bag from light. The infusion bag should be covered during administration to the subject until dosing is complete. It is not necessary to cover the infusion tubing or to use light-protective tubing during the infusion. • An infusion pump may be used. • Do not mix Trodelvy, or administer as an infusion, with other medicinal products. • Upon completion of the infusion, flush the intravenous line with 20 mL sodium chloride 9 mg/mL (0.9%) solution for injection. Disposal Any unused medicinal product or waste material should be disposed of in accordance with local requirements.

פרטי מסגרת הכללה בסל
א. התרופה תינתן כמונותרפיה לטיפול בסרטן שד לא נתיח או גרורתי, מסוג TNBC (Triple negative breast cancer), שקיבלו שני טיפולים סיסטמיים קודמים, שלפחות אחד מהם ניתן למחלה בשלב מתקדם. ב. מתן התרופה האמורה ייעשה לפי מרשם של רופא מומחה באונקולוגיה.
מסגרת הכללה בסל
התוויות הכלולות במסגרת הסל
| התוויה | תאריך הכללה | תחום קליני | Class Effect | מצב מחלה |
|---|---|---|---|---|
| א. התרופה תינתן כמונותרפיה לטיפול בסרטן שד לא נתיח או גרורתי, מסוג TNBC (Triple negative breast cancer), שקיבלו שני טיפולים סיסטמיים קודמים, שלפחות אחד מהם ניתן למחלה בשלב מתקדם. ב. מתן התרופה האמורה ייעשה לפי מרשם של רופא מומחה באונקולוגיה. | 17/03/2024 | אונקולוגיה | סרטן שד מסוג Triple negative, TNBC |
שימוש לפי פנקס קופ''ח כללית 1994
לא צוין
תאריך הכללה מקורי בסל
17/03/2024
הגבלות
תרופה מוגבלת לרישום ע'י רופא מומחה או הגבלה אחרת
ATC
מידע נוסף
