Quest for the right Drug
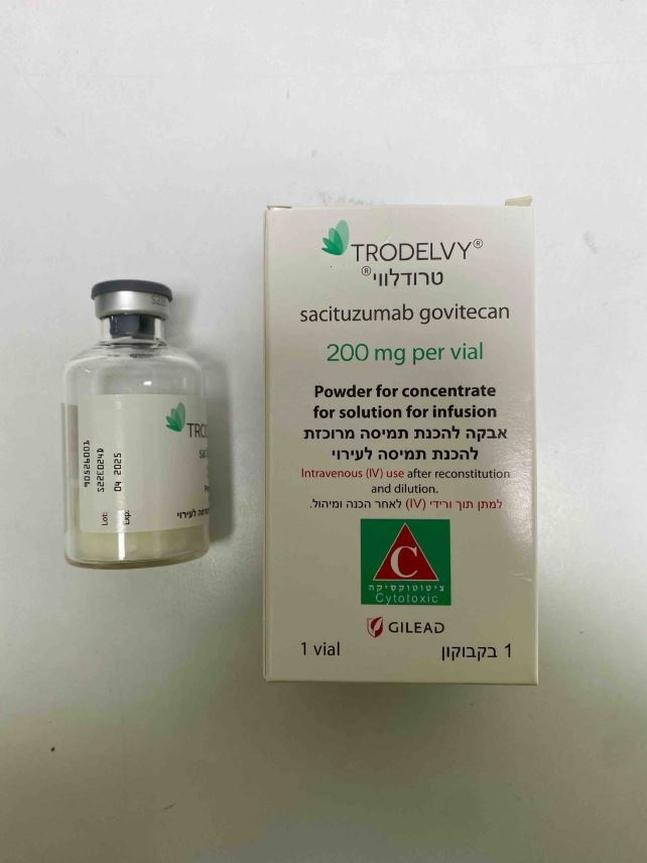
טרודלווי TRODELVY (SACITUZUMAB GOVITECAN)
תרופה במרשם
תרופה בסל
נרקוטיקה
ציטוטוקסיקה
צורת מתן:
תוך-ורידי : I.V
צורת מינון:
אין פרטים : POWDER FOR CONCENTRATE FOR SOLUTION FOR INFUSION
עלון לרופא
מינוניםPosology התוויות
Indications תופעות לוואי
Adverse reactions התוויות נגד
Contraindications אינטראקציות
Interactions מינון יתר
Overdose הריון/הנקה
Pregnancy & Lactation אוכלוסיות מיוחדות
Special populations תכונות פרמקולוגיות
Pharmacological properties מידע רוקחי
Pharmaceutical particulars אזהרת שימוש
Special Warning עלון לרופא
Physicians Leaflet
Posology : מינונים
4.2 Posology and method of administration Trodelvy must only be prescribed and administered to patients by healthcare professionals experienced in the use of anti-cancer therapies and administered in an environment where full resuscitation facilities are available. Posology The recommended dose of sacituzumab govitecan is 10 mg/kg body weight administered as an intravenous infusion once weekly on Day 1 and Day 8 of 21-day treatment cycles. Treatment should be continued until disease progression or unacceptable toxicity. Prevention treatment Prior to each dose of sacituzumab govitecan, treatment for prevention of infusion-related reactions and prevention of chemotherapy-induced nausea and vomiting (CINV) is recommended (see section 4.4). Dose modifications for infusion-related reactions The infusion rate of sacituzumab govitecan should be slowed down or infusion interrupted if the patient develops an infusion-related reaction. Sacituzumab govitecan should be permanently discontinued if life-threatening infusion-related reactions occur (see section 4.4). Dose modifications for adverse reactions Dose modifications to manage adverse reactions of sacituzumab govitecan are described in Table 1. The sacituzumab govitecan dose should not be re-escalated after a dose reduction for adverse reactions has been made. Table 1: Recommended dose modifications for adverse reactions Adverse reaction Occurrence Dose modification Severe neutropenia Grade 4 neutropenia ≥ 7 days or less if clinically indicated, First Administer granulocyte- OR colony stimulating factor Grade 3-4 febrile neutropenia (GCSF), as soon as OR clinically indicated At time of scheduled treatment, Grade 3-4 neutropenia which Second 25% dose reduction: delays dosing by 2 or 3 weeks for recovery to ≤ Grade 1 administer G-CSF as soon as clinically indicated Third 50% dose reduction: administer G-CSF as soon as clinically indicated Fourth Discontinue treatment: administer G-CSF as soon as clinically indicated At time of scheduled treatment, Grade 3-4 neutropenia which First Discontinue treatment: delays dosing beyond 3 weeks for recovery to ≤ Grade 1 administer G-CSF as soon as clinically indicated Severe non-neutropenic toxicity Grade 4 non-hematologic toxicity of any duration, First 25% dose reduction OR Second 50% dose reduction Any Grade 3-4 nausea, vomiting or diarrhoea due to treatment Third Discontinue treatment that is not controlled with antiemetics and anti-diarrhoeal agents, OR Other Grade 3-4 non-hematologic toxicity persisting > 48 hours despite optimal medical management, OR At time of scheduled treatment, Grade 3-4 non-neutropenic hematologic or non-hematologic toxicity, which delays dose by 2 or 3 weeks for recovery to ≤ Grade 1 In the event of Grade 3-4 non-neutropenic hematologic or non- First Discontinue treatment hematologic toxicity, Grade 3 nausea or Grade 3-4 vomiting, which does not recover to ≤ Grade 1 within 3 weeks Special populations Elderly No overall differences in efficacy of sacituzumab govitecan were observed in patients ≥ 65 and < 65 years old. No dose adjustment is required in patients ≥ 65 years old. Data from sacituzumab govitecan in patients ≥ 75 years are limited. Hepatic impairment No adjustment to the starting dose is required when administering sacituzumab govitecan to patients with mild hepatic impairment (bilirubin ≤ 1.5 upper limit of normal [ULN] and aspartate aminotransferase [AST]/alanine aminotransferase [ALT] < 3 ULN). The safety of sacituzumab govitecan in patients with moderate or severe hepatic impairment has not been established. Sacituzumab govitecan has not been studied in patients with any of the following: serum bilirubin > 1.5 ULN, or AST or ALT > 3 ULN in patients without liver metastases, or AST or ALT > 5 ULN in patients with liver metastases. The use of sacituzumab govitecan should be avoided in these patients. Renal impairment No adjustment to the starting dose is required when administering sacituzumab govitecan to patients with mild or moderate renal impairment. Sacituzumab govitecan has not been studied in patients with severe renal impairment or end-stage renal disease (Creatinine Clearance [CrCl] ≤ 15 mL/min). Paediatric population The safety and efficacy of sacituzumab govitecan in children aged 0 to 18 years have not been established. No data are available. Method of administration Sacituzumab govitecan is for intravenous use only. It must be administered as an intravenous infusion, not as an intravenous push or bolus. First infusion: the infusion should be administered over a period of 3 hours. Subsequent infusions: the infusion should be administered over a period of 1 to 2 hours if prior infusions were tolerated. Patients have to be observed during each infusion and for at least 30 minutes after each infusion for signs or symptoms of infusion-related reactions (see section 4.4). For instructions on reconstitution of the medicinal product before administration, see section 6.6.

פרטי מסגרת הכללה בסל
א. התרופה תינתן כמונותרפיה לטיפול בסרטן שד לא נתיח או גרורתי, מסוג TNBC (Triple negative breast cancer), שקיבלו שני טיפולים סיסטמיים קודמים, שלפחות אחד מהם ניתן למחלה בשלב מתקדם. ב. מתן התרופה האמורה ייעשה לפי מרשם של רופא מומחה באונקולוגיה.
מסגרת הכללה בסל
התוויות הכלולות במסגרת הסל
| התוויה | תאריך הכללה | תחום קליני | Class Effect | מצב מחלה |
|---|---|---|---|---|
| א. התרופה תינתן כמונותרפיה לטיפול בסרטן שד לא נתיח או גרורתי, מסוג TNBC (Triple negative breast cancer), שקיבלו שני טיפולים סיסטמיים קודמים, שלפחות אחד מהם ניתן למחלה בשלב מתקדם. ב. מתן התרופה האמורה ייעשה לפי מרשם של רופא מומחה באונקולוגיה. | 17/03/2024 | אונקולוגיה | סרטן שד מסוג Triple negative, TNBC |
שימוש לפי פנקס קופ''ח כללית 1994
לא צוין
תאריך הכללה מקורי בסל
17/03/2024
הגבלות
תרופה מוגבלת לרישום ע'י רופא מומחה או הגבלה אחרת
ATC
מידע נוסף
