Quest for the right Drug
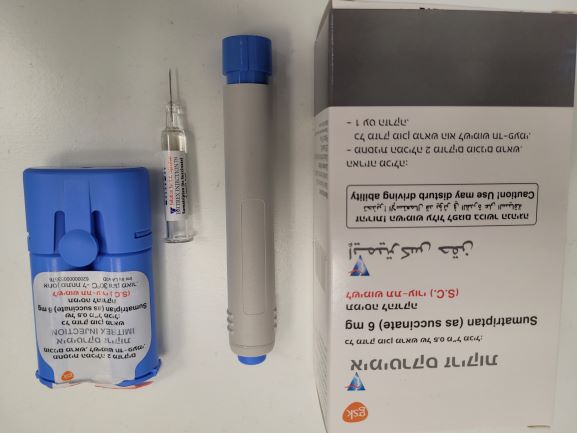
אימיטרקס זריקות IMITREX INJECTION (SUMATRIPTAN AS SUCCINATE)
תרופה במרשם
תרופה בסל
נרקוטיקה
ציטוטוקסיקה
צורת מתן:
תת-עורי : S.C
צורת מינון:
תמיסה להזרקה : SOLUTION FOR INJECTION
עלון לרופא
מינוניםPosology התוויות
Indications תופעות לוואי
Adverse reactions התוויות נגד
Contraindications אינטראקציות
Interactions מינון יתר
Overdose הריון/הנקה
Pregnancy & Lactation אוכלוסיות מיוחדות
Special populations תכונות פרמקולוגיות
Pharmacological properties מידע רוקחי
Pharmaceutical particulars אזהרת שימוש
Special Warning עלון לרופא
Physicians Leaflet
Pharmacological properties : תכונות פרמקולוגיות
Pharmacodynamic Properties
5.1 Pharmacodynamic properties Pharmacotherapeutic group: Analgesics: Selective 5-HT1 receptor agonists. ATC Code: N02CC01 Sumatriptan has been demonstrated to be a specific and selective 5- hydroxytryptamine (5-HT1D) receptor agonist with no effect on other 5-HT receptor (5-HT2-5-HT7) subtypes. The vascular 5-HT1D receptor is found predominantly in cranial blood vessels and mediates vasoconstriction. In animals, sumatriptan selectively constricts the carotid arterial circulation but does not alter cerebral blood flow. The carotid arterial circulation supplies blood to the extracranial and intracranial tissues, such as the meninges and dilatation and/or oedema formation in these vessels is thought to be the underlying mechanism of migraine in man. In addition, experimental evidence from animal studies suggests that sumatriptan inhibits trigeminal nerve activity. Both these actions (cranial vasoconstriction and inhibition of trigeminal nerve activity) may contribute to the anti-migraine action of sumatriptan in humans. Sumatriptan remains effective in treating menstrual migraine i.e. migraine without aura that occurs between 3 days prior and up to 5 days post onset of menstruation. Sumatriptan should be taken as soon as possible in an attack. Clinical response begins 10 to 15 minutes following a 6mg subcutaneous injection. Because of its route of administration Imitrex Injection may be particularly suitable for patients who suffer with nausea and vomiting during an attack.
Pharmacokinetic Properties
5.2 Pharmacokinetic properties Following subcutaneous injection, sumatriptan has a high mean bioavailability (96%) with peak serum concentrations occurring in 25 minutes. Average peak serum concentration after a 6 mg subcutaneous dose is 72 ng/ml. The elimination phase half life is approximately two hours. Plasma protein binding is low (14 to 21%), mean volume of distribution is 170 litres. Mean total plasma clearance is approximately 1160 ml/min and the mean renal plasma clearance is approximately 260 ml/min. Non-renal clearance accounts for about 80% of the total clearance. Sumatriptan is eliminated primarily by oxidative metabolism mediated by monoamine oxidase A. The major metabolite, the indole acetic acid analogue of sumatriptan, is mainly excreted in the urine where it is present as a free acid and the glucuronide conjugate. It has no known 5-HT1 or 5-HT2 activity. Minor metabolites have not been identified. In a pilot study no significant differences were found in the pharmacokinetic parameters between older people and young healthy volunteers. The effect of moderate hepatic disease (Child Pugh grade B) on the pharmacokinetics of subcutaneously administered sumatriptan has been evaluated. There were no significant differences in the pharmacokinetics of subcutaneously administered sumatriptan in moderately hepatically impaired subjects compared with healthy controls (see section 4.4).

מסגרת הכללה בסל
התוויות הכלולות במסגרת הסל
| התוויה | תאריך הכללה | תחום קליני | Class Effect | מצב מחלה |
|---|---|---|---|---|
| ZOLMITRIPTAN | ||||
| RIZATRIPTAN | ||||
| SUMATRIPTAN |
שימוש לפי פנקס קופ''ח כללית 1994
Acute migraine attacks with or without aura, cluster headache (s.c. injection only). יירשם ע"י רופא נוירולוג לפי פרוטוקול טיפולי מחייב
תאריך הכללה מקורי בסל
01/01/1995
הגבלות
תרופה מוגבלת לרישום ע'י רופא מומחה או הגבלה אחרת
מידע נוסף
