Quest for the right Drug
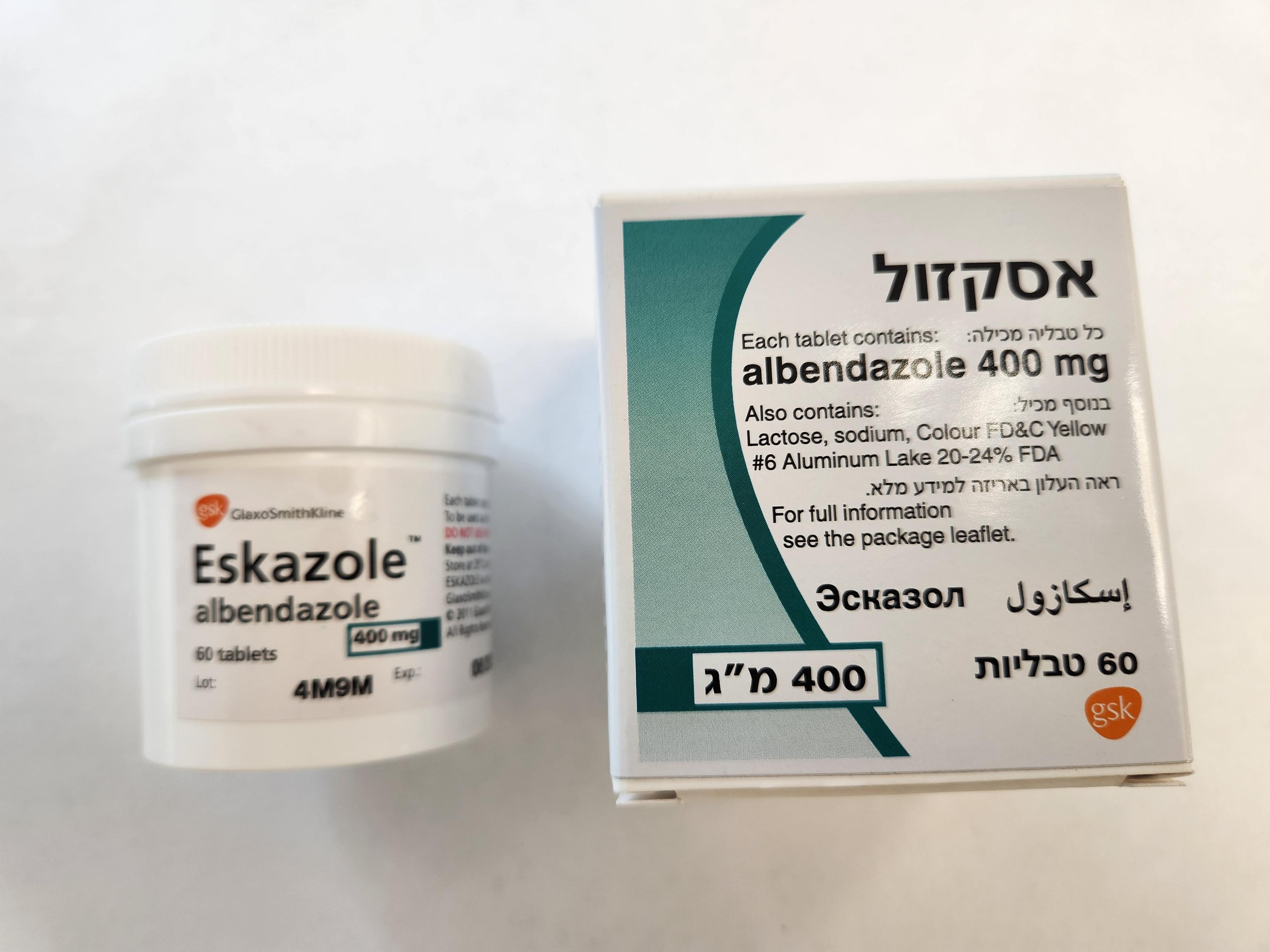
אסקזול ESKAZOLE (ALBENDAZOLE)
תרופה במרשם
תרופה בסל
נרקוטיקה
ציטוטוקסיקה
צורת מתן:
פומי : PER OS
צורת מינון:
טבליה : TABLETS
עלון לרופא
מינוניםPosology התוויות
Indications תופעות לוואי
Adverse reactions התוויות נגד
Contraindications אינטראקציות
Interactions מינון יתר
Overdose הריון/הנקה
Pregnancy & Lactation אוכלוסיות מיוחדות
Special populations תכונות פרמקולוגיות
Pharmacological properties מידע רוקחי
Pharmaceutical particulars אזהרת שימוש
Special Warning עלון לרופא
Physicians Leaflet
Posology : מינונים
4.2. Posology and method of administration Posology There is limited experience of use of albendazole in children under 6 years of age; therefore use in children under this age is not recommended. Dosages are dependent on the parasites involved, the weight of the patient, and the seriousness of the infection. • Cystic Echinococcosis Patients weighing > 60 kg: Total daily dose of 800 mg, given in two divided doses of 400 mg for a total of 28 days. Patients weighing < 60 kg: Total daily dose of 15 mg/kg given in two equally divided doses (maximum dose of 800 mg/day) for a total of 28 days. These 28-day cycles of treatment may be repeated after a 14-day period without treatment between cycles depending on the therapeutic indication, for a total of 3 cycles. 1. Inoperable and multiple cysts Up to three 28-day cycles of treatment with albendazole may be given for the treatment of liver, lung and peritoneal cysts. More prolonged treatment may be required for bone and brain locations. 2. Preoperative Two 28-day cycles should be given prior to surgery. Where surgical intervention is necessary before completion of two cycles, albendazole should be given for as long as possible. 3. Postoperative Where only a short preoperative course has been given (less than 14 days) and in those cases where emergency surgery is required, albendazole should be given postoperatively for two 28-day cycles separated by a 14-day period without treatment. Additionally, if cysts are viable following presurgical treatment or if spillage has occurred, a full two-cycle course should be given. 4. After percutaneous cyst drainage Similar treatment as for postoperative. • Alveolar echinococcosis Patients weighing > 60 kg: Total daily dose of 800 mg, given in two divided doses of 400 mg for 28-day cycles with 14-day periods without treatment between cycles. Patients weighing < 60 kg: Total daily dose of 15 mg/kg given in two equally divided doses (maximum dose of 800 mg/day) for 28-day cycles with 14-day periods without treatment between cycles. Treatment is given in 28-day cycles. It may be prolonged for months or even years. Continuous treatment at the same dose has been used for periods of up to 20 months. Current follow up suggests that survival times are substantially improved after prolonged treatment. It has been demonstrated in a limited number of patients that continuous treatment may lead to an apparent cure. • Elderly Experience in patients 65 years of age or older is limited. Reports indicate that no dose adjustment is required; however, albendazole should be used with caution in elderly patients with evidence of hepatic dysfunction (see section 4.2 “Hepatic impairment” and section 5.2). • Renal impairment Since renal excretion of albendazole and its primary metabolite, albendazole sulfoxide, is negligible, it is unlikely that clearance of these compounds would be altered in these patients. No dose adjustment is required; however, patients with evidence of renal impairment should be carefully monitored. • Hepatic impairment Since albendazole is rapidly metabolized by the liver to its primary pharmacologically active metabolite, albendazole sulfoxide, hepatic impairment would be expected to have significant effects on the pharmacokinetics of albendazole sulfoxide. Patients with abnormal hepatic function test results (transaminases) prior to starting treatment with albendazole should be carefully evaluated and treatment should be discontinued if hepatic enzymes are significantly increased or full blood count decreases to a clinically significant level (see sections 4.4 and 4.8). Method of administration Eskazole should be taken with meals (see section 5.2). Some people, particularly young children, may experience difficulties swallowing the tablets whole and should be encouraged to chew them with a little water or alternatively, they may be crushed.

שימוש לפי פנקס קופ''ח כללית 1994
לא צוין
תאריך הכללה מקורי בסל
לא צוין
הגבלות
לא צוין
מידע נוסף
עלון מידע לצרכן
03.05.18 - עלון לצרכן 27.02.20 - עלון לצרכן אנגלית 27.02.20 - עלון לצרכן עברית 27.02.20 - עלון לצרכן ערבית 03.01.23 - עלון לצרכן עברית 05.06.23 - עלון לצרכן אנגלית 05.06.23 - עלון לצרכן עברית 05.06.23 - עלון לצרכן ערבית 10.10.24 - עלון לצרכן עברית 30.11.11 - החמרה לעלון 19.04.17 - החמרה לעלוןלתרופה במאגר משרד הבריאות
אסקזול