Quest for the right Drug
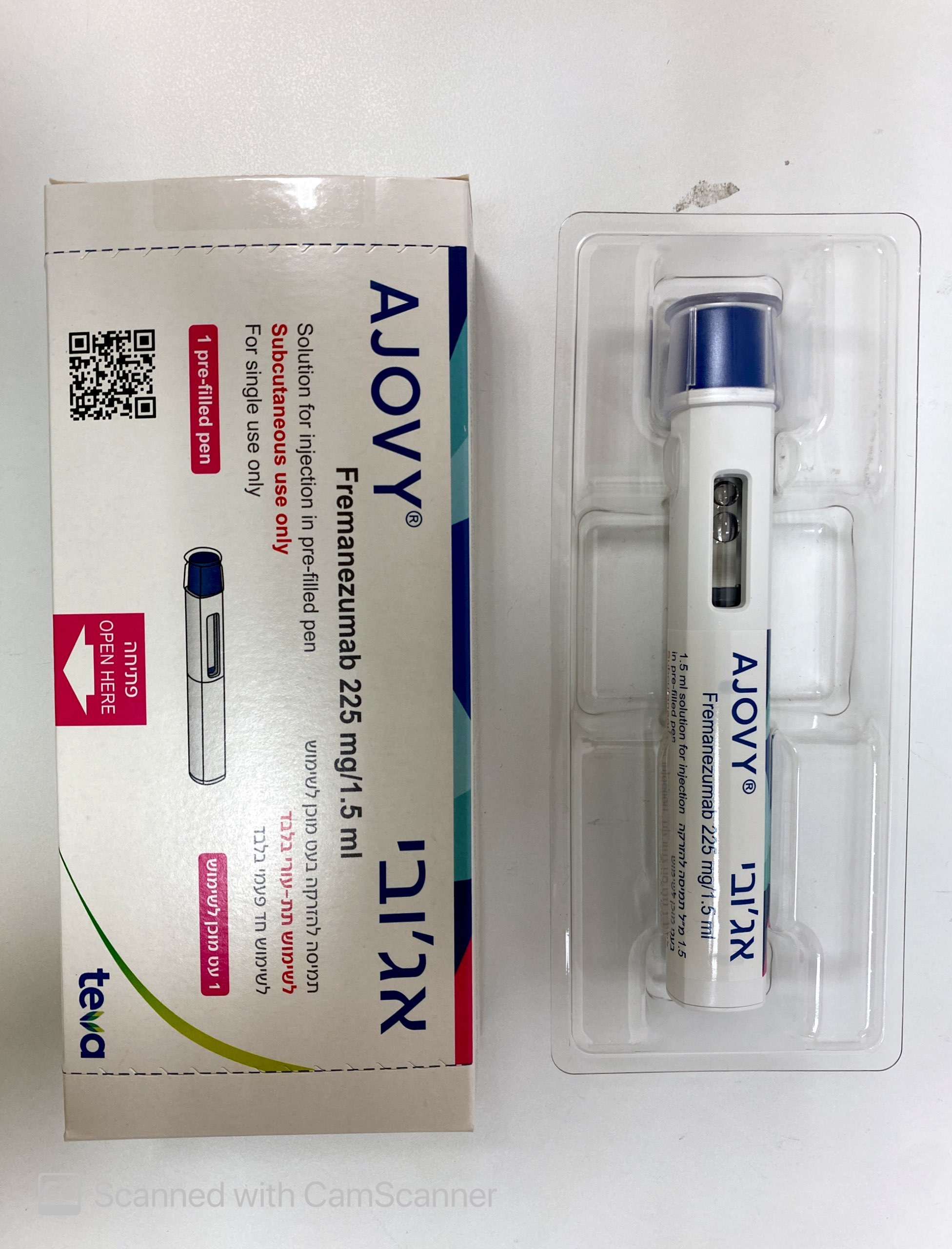
אג'ובי AJOVY (FREMANEZUMAB)
תרופה במרשם
תרופה בסל
נרקוטיקה
ציטוטוקסיקה
צורת מתן:
תת-עורי : S.C
צורת מינון:
תמיסה להזרקה : SOLUTION FOR INJECTION
עלון לרופא
מינוניםPosology התוויות
Indications תופעות לוואי
Adverse reactions התוויות נגד
Contraindications אינטראקציות
Interactions מינון יתר
Overdose הריון/הנקה
Pregnancy & Lactation אוכלוסיות מיוחדות
Special populations תכונות פרמקולוגיות
Pharmacological properties מידע רוקחי
Pharmaceutical particulars אזהרת שימוש
Special Warning עלון לרופא
Physicians Leaflet
Adverse reactions : תופעות לוואי
4.8 Undesirable effects Summary of the safety profile A total of over 2,500 patients (more than 1,900 patient years) have been treated with AJOVY in registration studies. More than 1,400 patients were treated for at least 12 months. Commonly reported adverse drug reactions (ADRs) were local reactions at the injection site (pain [24%], induration [17%], erythema [16%] and pruritus [2%]). Tabulated list of adverse reactions ADRs from clinical studies and post-marketing reports are presented according to MedDRA system organ classification. Within each each frequency grouping, ADRs are presented in the order of decreasing seriousness. Frequency categories are based on the following convention: very common (≥1/10); common (≥1/100 to <1/10); uncommon (≥1/1,000 to <1/100); rare (≥1/10,000 to <1/1,000); very rare (<1/10,000). Within each system organ class, ADRs are ranked by frequency, most frequent reactions first. The following ADRs have been identified for AJOVY (Table 1). Table 1: Adverse reactions MedDRA System Organ Frequency Adverse Reaction Class Immune system disorders Uncommon Hypersensitivity reactions such as rash, pruritus, urticaria and swelling Rare Anaphylactic reaction General disorders and Very common Injection site pain administration site conditions Injection site induration Injection site erythema Common Injection site pruritus Uncommon Injection site rash Description of selected adverse reactions Injection site reactions The most frequently observed local reactions at the injection site were pain, induration and erythema. All local injection site reactions were transient and predominantly mild to moderate in severity. Pain, induration and erythema were typically observed immediately after injection while pruritus and rash appeared within a median of 24 and 48 hours, respectively. All injection site reactions resolved, mostly within a few hours or days. Injection site reactions generally did not necessitate discontinuation of the medicinal product. Serious hypersensitivity reactions Anaphylactic reactions have been reported rarely. These reactions mostly occurred within 24 hours of administration although some reactions have been delayed. Immunogenicity In placebo-controlled studies, 0.4 % of patients (6 out of 1,701) treated with fremanezumab developed anti-drug antibodies (ADA). The antibody responses were of low titer. One of these 6 patients developed neutralising antibodies. With 12 months of treatment ADA were detected in 2.3% of the patients (43 out of 1,888) with 0.95% of the patients developing neutralising antibodies. The safety and efficacy of fremanezumab were not affected by ADA development. Reporting of suspected adverse reactions Reporting suspected adverse reactions after authorisation of the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the medicinal product. Any suspected adverse events should be reported to the Ministry of Health according to the National Regulation by using an online form https://sideeffects.health.gov.il

שימוש לפי פנקס קופ''ח כללית 1994
לא צוין
תאריך הכללה מקורי בסל
לא צוין
הגבלות
לא צוין
ATC
מידע נוסף
עלון מידע לצרכן
02.11.20 - עלון לצרכן אנגלית 01.03.22 - עלון לצרכן עברית 01.03.22 - עלון לצרכן עברית 02.11.20 - עלון לצרכן ערבית 03.01.23 - עלון לצרכן אנגלית 03.01.23 - עלון לצרכן עברית 03.01.23 - עלון לצרכן ערבית 07.06.23 - עלון לצרכן עברית 07.06.23 - עלון לצרכן עברית 24.08.23 - עלון לצרכן אנגלית 24.08.23 - עלון לצרכן עברית 24.08.23 - עלון לצרכן ערבית 07.12.20 - החמרה לעלון 01.03.22 - החמרה לעלוןלתרופה במאגר משרד הבריאות
אג'ובי