Quest for the right Drug
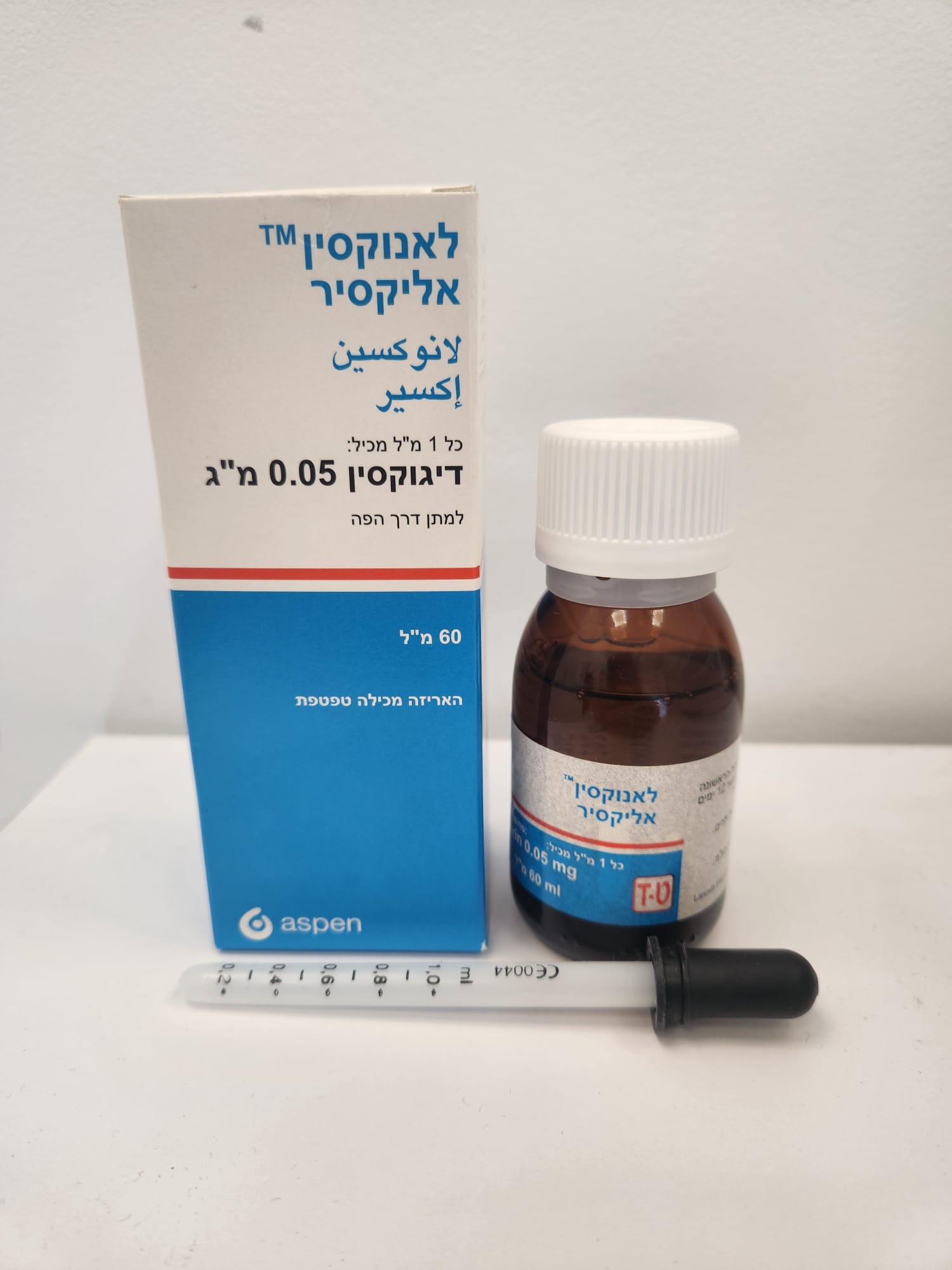
לאנוקסין אליקסיר LANOXIN ELIXIR (DIGOXIN)
תרופה במרשם
תרופה בסל
נרקוטיקה
ציטוטוקסיקה
צורת מתן:
פומי : PER OS
צורת מינון:
תמיסה אלכוהולית : ELIXIR
עלון לרופא
מינוניםPosology התוויות
Indications תופעות לוואי
Adverse reactions התוויות נגד
Contraindications אינטראקציות
Interactions מינון יתר
Overdose הריון/הנקה
Pregnancy & Lactation אוכלוסיות מיוחדות
Special populations תכונות פרמקולוגיות
Pharmacological properties מידע רוקחי
Pharmaceutical particulars אזהרת שימוש
Special Warning עלון לרופא
Physicians Leaflet
Adverse reactions : תופעות לוואי
4.8. Undesirable effects Summary of the safety profile In general, the adverse reactions of digoxin are dose-dependent and occur at doses higher than those needed to achieve a therapeutic effect. Hence, adverse reactions are less common when digoxin is used within the recommended dose range or therapeutic serum concentration range and when there is careful attention to concurrent medications and conditions. Tabulated list of adverse reactions Adverse reactions are listed below by system organ class and frequency. Frequencies are defined as: Very common ≥ 1/10 Common ≥ 1/100 and < 1/10 Uncommon ≥ 1/1000 and < 1/100 Rare ≥ 1/10,000 and < 1/1000 Very rare < 1/10,000, including isolated reports. Very common, common and uncommon events were generally determined from clinical trial data. The incidence in placebo was taken into account. Adverse drug reactions identified through post- marketing surveillance were considered to be rare or very rare (including isolated reports). System Organ Class Frequency Side effects Blood and lymphatic system Very rare Thrombocytopaenia disorders Metabolism and nutrition disorders Very rare Decreased appetite Psychiatric disorders Uncommon Depression Very rare Psychotic disorder, apathy, confusional state Nervous system disorders Common Nervous system disorder, dizziness Very rare Headache Eye disorders Common Visual impairment (blurred vision or xanthopsia) Cardiac disorders Common Arrhythmia, conduction disorder, bigeminy, trigeminy, PR prolongation, sinus bradycardia Very rare Supraventricular tachyarrhythmia, atrial tachycardia (with or without block), supraventricular tachycardia (nodal arrhythmia), ventricular arrhythmia, ventricular extrasystoles, electrocardiogram ST segment depression Gastrointestinal disorders Common Nausea, vomiting, diarrhoea Very rare Intestinal ischaemia, gastrointestinal necrosis Skin and subcutaneous tissue Common Rash* disorders Reproductive system and breast Very rare Gynaecomastia* disorders General disorders and administration Very rare Fatigue, malaise, asthenia site conditions *See “Description of selected adverse reactions” Description of selected adverse reactions Skin and subcutaneous tissue disorders Skin rashes of urticarial or scarlatiniform character may be accompanied by pronounced eosinophilia. Reproductive system and breast disorders Gynaecomastia can occur with long term administration. Reporting of suspected adverse reactions Reporting suspected adverse reactions after authorisation of the medicinal product is important. It allows continued monitoring of the benefit / risk balance of the medicinal product. Any suspected adverse events should be reported to the Ministry of Health according to the National Regulation by using an online form http://sideeffects.health.gov.il Additionally, you can also report to Padagis via the following address: Padagis.co.il

שימוש לפי פנקס קופ''ח כללית 1994
Congestive heart failure, pulmonary edema, acute and chronic atrial fibrillation and flutter, other supraventricular arrhythmias
תאריך הכללה מקורי בסל
01/01/1995
הגבלות
תרופה שאושרה לשימוש כללי בקופ'ח
מידע נוסף
