Quest for the right Drug
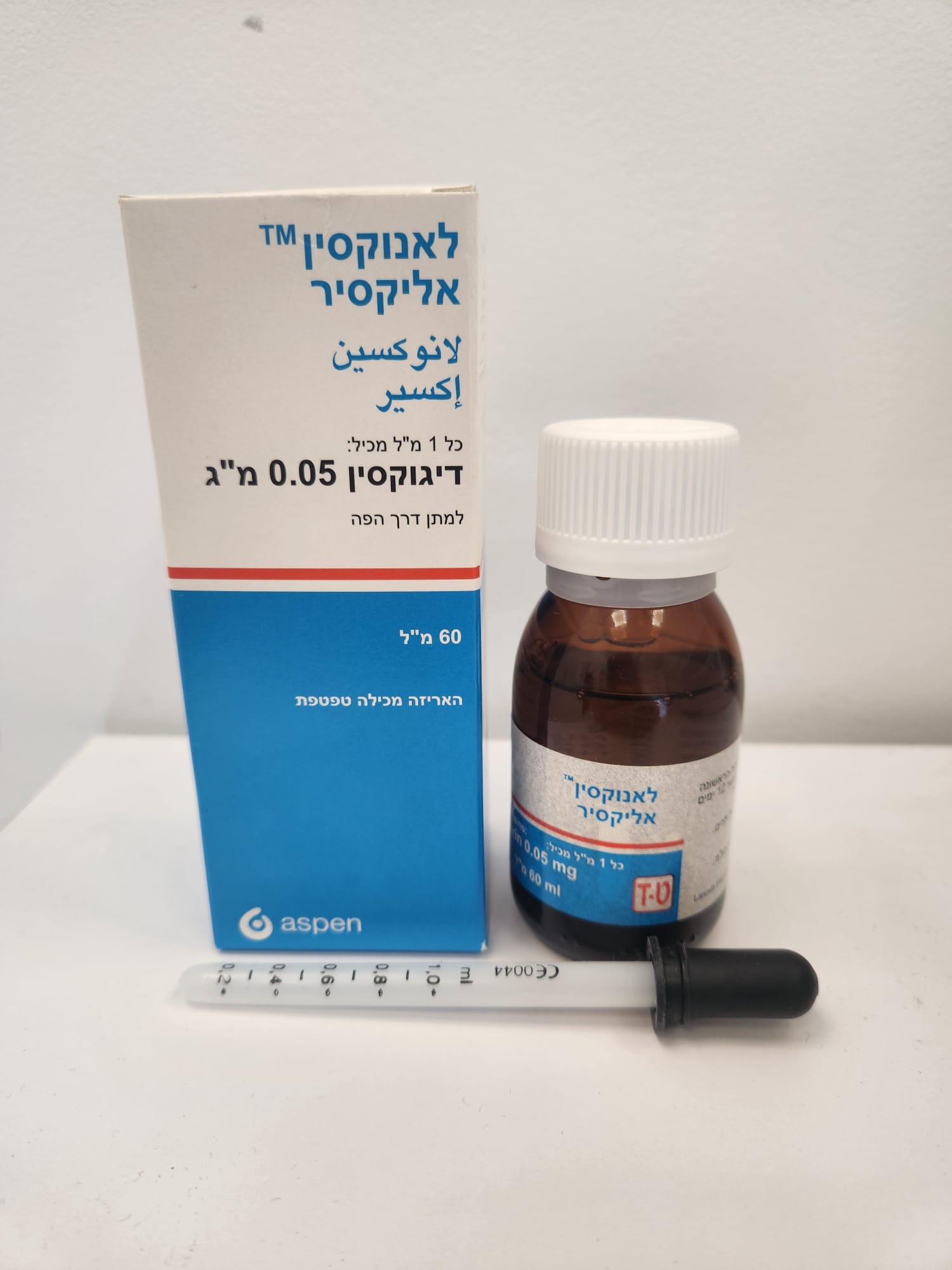
לאנוקסין אליקסיר LANOXIN ELIXIR (DIGOXIN)
תרופה במרשם
תרופה בסל
נרקוטיקה
ציטוטוקסיקה
צורת מתן:
פומי : PER OS
צורת מינון:
תמיסה אלכוהולית : ELIXIR
עלון לרופא
מינוניםPosology התוויות
Indications תופעות לוואי
Adverse reactions התוויות נגד
Contraindications אינטראקציות
Interactions מינון יתר
Overdose הריון/הנקה
Pregnancy & Lactation אוכלוסיות מיוחדות
Special populations תכונות פרמקולוגיות
Pharmacological properties מידע רוקחי
Pharmaceutical particulars אזהרת שימוש
Special Warning עלון לרופא
Physicians Leaflet
Special Warning : אזהרת שימוש
4.4. Special warnings and precautions for use Monitoring Patients receiving digoxin should have their serum electrolytes and renal function (serum creatinine concentration) assessed periodically; the frequency of assessments will depend on the clinical setting. Serum concentrations of digoxin may be expressed in Conventional Units of nanograms/ml or SI Units of nanomol/l. To convert nanograms/ml to nanomol/l, multiply nanograms/ml by 1.28. The serum concentration of digoxin can be determined by radioimmunoassay. Blood should be taken six hours or more after the last dose of digoxin. There are no rigid guidelines as to the range of serum concentrations that are most efficacious. Post hoc analyses of heart failure patients in the Digitalis Investigation Group trial suggest that the optimal trough digoxin serum level may be 0.5 nanogram/ml (0.64 nanomol/l) to 1.0 nanogram/ml (1.28 nanomol/l). Digoxin toxicity is more commonly associated with serum digoxin concentrations greater than 2 nanogram/ml. However, serum digoxin concentration should be interpreted in the clinical context. Toxicity may occur with lower digoxin serum concentrations. In deciding whether a patient's symptoms are due to digoxin, the clinical state together with the serum potassium level and thyroid function are important factors (see Section 4.9). Determination of the serum digoxin concentration may be very helpful in making a decision to treat with further digoxin, but other glycosides and endogenous digoxin-like substances, including metabolites of digoxin, can interfere with the assays that are available and one should always be wary of values which do not seem commensurate with the clinical state of the patient. Observations while temporary withholding digoxin might be more appropriate Arrhythmias Arrhythmias may be precipitated by digoxin toxicity, some of which can resemble arrhythmias for which the drug could be advised. For example, atrial tachycardia with varying atrioventricular block requires particular care as clinically the rhythm resembles atrial fibrillation. Many beneficial effects of digoxin on arrhythmias result from a degree of atrioventricular conduction blockade. However, when incomplete atrioventricular block already exists the effects of a rapid progression in the block should be anticipated. In complete heart block the idioventricular escape rhythm may be suppressed. Sinoatrial disorder In some cases of sinoatrial disorder (i.e. Sick Sinus Syndrome) digoxin may cause or exacerbate sinus bradycardia or cause sinoatrial block. Myocardial infarction The administration of digoxin in the period immediately following myocardial infarction is not contraindicated. However, the use of inotropic drugs in some patients in this setting may result in undesirable increases in myocardial oxygen demand and ischaemia, and some retrospective follow-up studies have suggested digoxin to be associated with an increased risk of death. The possibility of arrhythmias arising in patients who may be hypokalaemic after myocardial infarction and are likely to be haemodynamically unstable must be borne in mind. The limitations imposed thereafter on direct current cardioversion must also be remembered. Cardiac amyloidosis Treatment with digoxin should generally be avoided in patients with heart failure associated with cardiac amyloidosis. However, if alternative treatments are not appropriate, digoxin can be used to control the ventricular rate in patients with cardiac amyloidosis and atrial fibrillation. Myocarditis Digoxin can rarely precipitate vasoconstriction and therefore should be avoided in patients with myocarditis. Beri-beri heart disease Patients with beri-beri heart disease may fail to respond adequately to digoxin if the underlying thiamine deficiency is not treated concomitantly. Constrictive pericarditis Digoxin should not be used in constrictive pericarditis unless it is used to control the ventricular rate in atrial fibrillation or to improve systolic dysfunction. Exercise tolerance Digoxin improves exercise tolerance in patients with impaired left ventricular systolic dysfunction and normal sinus rhythm. This may or may not be associated with an improved haemodynamic profile. However, the benefit of digoxin in patients with supraventricular arrhythmias is most evident at rest, less evident with exercise. Withdrawal In patients receiving diuretics and an ACE inhibitor, or diuretics alone, the withdrawal of digoxin has been shown to result in clinical deterioration. Electrocardiograhy The use of therapeutic doses of digoxin may cause prolongation of the PR interval and depression of the ST segment on the electrocardiogram. Digoxin may produce false positive ST-T changes on the electrocardiogram during exercise testing. These electrophysiologic effects reflect an expected effect of the drug and are not indicative of toxicity. Severe respiratory disease Patients with severe respiratory disease may have an increased myocardial sensitivity to digitalis glycosides. Hypokalaemia Hypokalaemia sensitises the myocardium to the actions of cardiac glycosides. Hypoxia, hypomagnesaemia and hypercalcaemia Hypoxia, hypomagnesaemia and marked hypercalcaemia increase myocardial sensitivity to cardiac glycosides. Thyroid disease Administering digoxin to a patient with thyroid disease requires care. Initial and maintenance doses of digoxin should be reduced when thyroid function is subnormal. In hyperthyroidism there is relative digoxin resistance and the dose may have to be increased. During the course of treatment of thyrotoxicosis, dosage should be reduced as the thyrotoxicosis comes under control. Malabsorption Patients with malabsorption syndrome or gastro-intestinal reconstructions may require larger doses of digoxin. Chronic congestive cardiac failure Although many patients with chronic congestive cardiac failure benefit from acute administration of digoxin, there are some in whom it does not lead to constant, marked or lasting haemodynamic improvement. It is therefore important to evaluate the response of each patient individually when digoxin is continued long-term. Direct current cardioversion: The risk of provoking dangerous arrhythmias with direct current cardioversion is greatly increased in the presence of digitalis toxicity and is in proportion to the cardioversion energy used. For elective direct current cardioversion of a patient who is taking digoxin, the drug should be withheld for 24 hours before cardioversion is performed. In emergencies, such as cardiac arrest when attempting cardioversion the lowest effective energy should be applied. Direct current cardioversion is inappropriate in the treatment of arrhythmias thought to be caused by cardiac glycosides. Lanoxin Elixir contains sucrose. Patients with rare hereditary problems of fructose intolerance, glucose-galactose malabsorption or sucrase-isomaltase insufficiency should not take this medicine. Excipients warnings Methyl parahydroxybenzoate (E218) • Lanoxin Elixir contains methyl parahydroxybenzoate (E218) which may cause allergic reactions (possibly delayed). Sucrose • Lanoxin Elixir contains less 0.3 g of sucrose in each ml of oral solution, i.e. 1.5 g of sucrose in a 5 ml (0.25 mg digoxin) dose. Patients with rare hereditary problems of fructose intolerance, glucose-galactose malabsorption or sucrase-isomaltase insufficiency should not take this medicine. Ethanol • Lanoxin Elixir contains less than 0.1 ml of ethanol (alcohol) in each ml of oral solution, i.e. up to 0.44 g of ethanol in a 5 ml (0.25 mg digoxin) dose which is equivalent to less than 12.5 ml (less than 3 teaspoons) beer, less than 4.5 ml (less than one teaspoon) wine per 0.25 mg digoxin dose. Harmful to those suffering from alcoholism. To be taken into account in pregnant or breast-feeding woman, children and high-risk groups such as patients with liver disease, or epilepsy. Sodium • Adults and children over 10 years: This medicinal product contains 38.019 mg sodium or less per dose, equivalent to 1.9 % of the WHO recommended maximum daily intake of 2 g sodium for an adult.
Effects on Driving

שימוש לפי פנקס קופ''ח כללית 1994
Congestive heart failure, pulmonary edema, acute and chronic atrial fibrillation and flutter, other supraventricular arrhythmias
תאריך הכללה מקורי בסל
01/01/1995
הגבלות
תרופה שאושרה לשימוש כללי בקופ'ח
מידע נוסף
