Quest for the right Drug
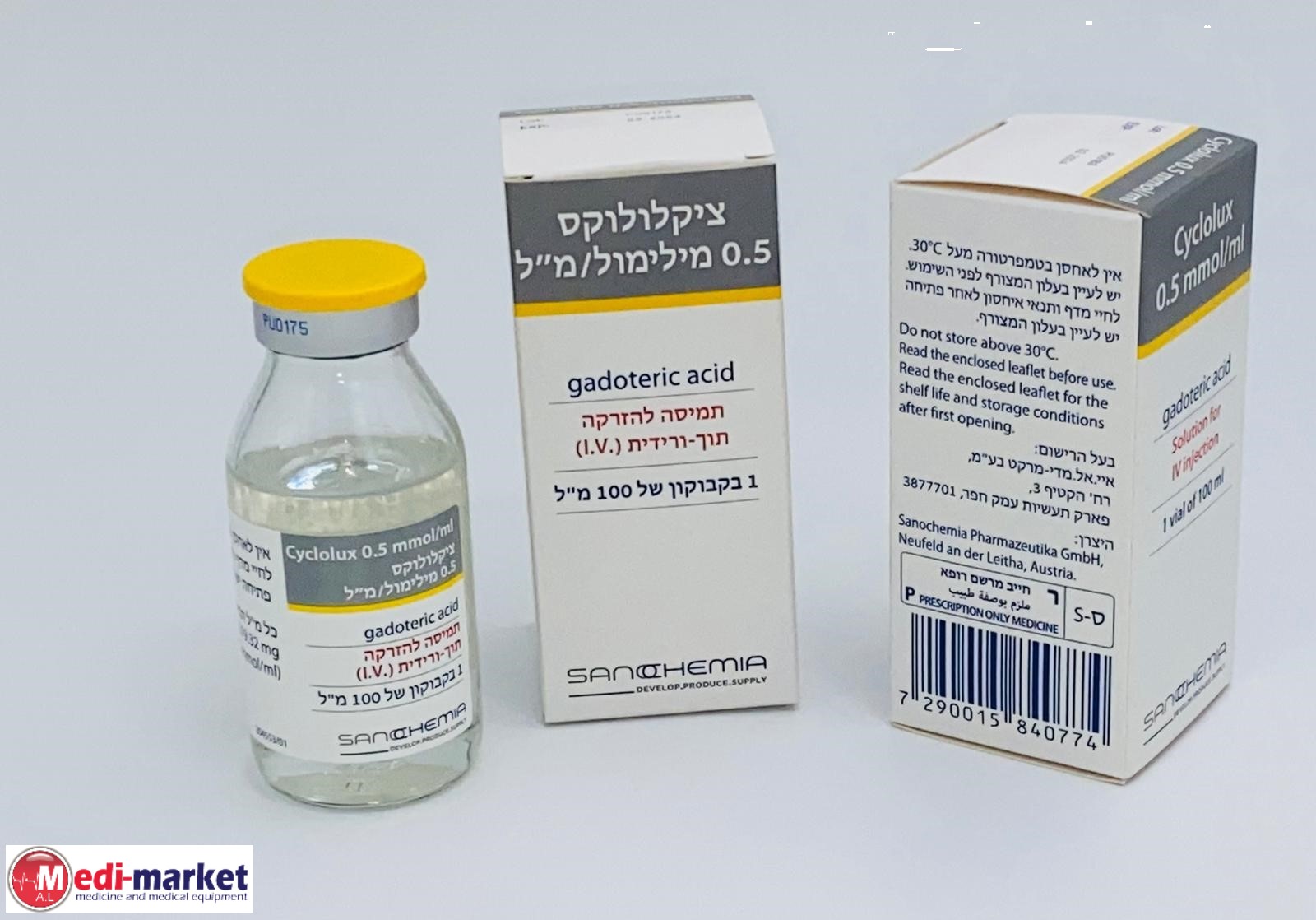
ציקלולוקס 0.5 מילימול / מ"ל CYCLOLUX 0.5 MMOL/ML (GADOTERIC ACID)
תרופה במרשם
תרופה בסל
נרקוטיקה
ציטוטוקסיקה
צורת מתן:
תוך-ורידי : I.V
צורת מינון:
תמיסה להזרקה : SOLUTION FOR INJECTION
עלון לרופא
מינוניםPosology התוויות
Indications תופעות לוואי
Adverse reactions התוויות נגד
Contraindications אינטראקציות
Interactions מינון יתר
Overdose הריון/הנקה
Pregnancy & Lactation אוכלוסיות מיוחדות
Special populations תכונות פרמקולוגיות
Pharmacological properties מידע רוקחי
Pharmaceutical particulars אזהרת שימוש
Special Warning עלון לרופא
Physicians Leaflet
Adverse reactions : תופעות לוואי
4.8 Undesirable effects Side effects in association with the use of gadoteric acid are usually mild to moderate in intensity and transient in nature. Injection site reactions, nausea and headache are the most frequently observed reactions. During clinical trials uncommon adverse reactions (≥1/1000 to <1/100) were nausea, headache, injection site reactions, feeling cold, hypotension, somnolence, dizziness, feeling hot, burning sensation, rash, asthenia, dysgeusia and hypertension were the most frequent. Post-marketing the most commonly reported adverse reactions following administration of gadoteric acid have been nausea, vomiting, pruritus and hypersensitivity reactions. In hypersensitivity reactions, the reactions most frequently observed are skin reactions, which can be localised, extended or generalised. These reactions occur most often immediately (during the injection or within one hour after the start of injection) or sometimes delayed (one hour to several days after injection), presenting as skin reactions in this case. Immediate reactions include one or more effects, which appear simultaneously or sequentially, and are most often cutaneous, respiratory, gastrointestinal, joint and/or cardiovascular reactions. Each sign maybe a warning sign of shock and may very rarely lead to death. Isolated cases of nephrogenic systemic fibrosis (NSF) have been reported with gadoteric acid, most of which were in patients co-administered other gadolinium- containing contrast agents (see section 4.4). The adverse reactions are listed in the table below by SOC (System Organ Class) and by frequency according to the following categories: very common (1/10), common (1/100 to 1<1/10), uncommon (1/1000 to 1<1/100), rare (1/10 000 to <1/1 000), very rare (<1/10 000), not known (cannot be estimated on the basis of available data). The data presented are from clinical trials involving 2822 patients when available, or from a pool of observational studies involving 185,500 patients. System Organ Class Frequency: adverse reaction Immune system Uncommon: hypersensitivity disorders Very rare: anaphylactic reaction, anaphylactoid reaction Psychiatric Rare: anxiety disorders Very rare: agitation Nervous system Uncommon: headache, dysgeusia, dizziness, drowsiness, paraesthesia disorders (including burning sensation) Rare: presyncope Very rare: coma, convulsion, syncope, tremor, parosmia Eye disorders Rare: eyelid oedema Very rare: conjunctivitis, ocular hyperaemia, blurred vision, excess tears Cardiac disorders Rare: palpitations Very rare: tachycardia, cardiac arrest, arrhythmia, bradycardia Vascular disorders Uncommon: hypotension, hypertension, Very rare: pallor, vasodilatation Respiratory, Rare: sneezing thoracic and Very rare: cough, dyspnoea, nasal congestion, respiratory arrest, mediastinal bronchospasm, laryngospasm, pharyngeal oedema, dry throat, disorders pulmonary oedema Gastrointestinal Uncommon: nausea, abdominal pain disorders Rare: vomiting, diarrhoea, salivary hypersecretion Skin and subcutaneous Uncommon: rash tissue disorders Rare: urticaria, pruritushyperhidrosis Very rare: erythema, angioedema, eczema Not known: nephrogenic systemic fibrosis Musculoskeletal and Very rare: muscle cramps, muscular weakness, back pain connective tissue disorders General disorders and Uncommon: feeling hot, feeling cold, asthenia, injection site reactions administration site (extravasation, pain, discomfort, oedema, inflammation, coldness) conditions Rare: chest pain, chills Very rare: malaise, chest discomfort, pyrexia, face oedema, injection site necrosis (in case of extravasation), phlebitis superficial Investigations Very rare: decreased oxygen saturation The following adverse reactions were reported with other intravenous contrast agents for MRI: System Organ Class Adverse reaction Blood and lymphatic system Haemolysis disorders Psychiatric disorders Confusion Eye disorders Blindness transient, eye pain Ear and labyrinth disorders Tinnitus, ear pain Respiratory, thoracic and Asthma mediastinal disorders Gastrointestinal disorders Dry mouth Skin and subcutaneous tissue Dermatitis bullous disorders Renal and urinary disorders Urinary incontinence, renal tubular necrosis, acute renal failure Investigations Electrocardiogram PR prolongation, blood iron increased, blood bilirubin increased, serum ferritin increased, liver function test abnormal Adverse reactions in Children Safety of paediatric patients was considered in clinical trials and post-marketing studies. As compared to adults, the safety profile of gadoteric acid did not show any specificity in children. The most common reactions are gastrointestinal symptoms or signs of hypersensitivity. Reporting of suspected adverse reactions Reporting suspected adverse reactions after authorisation of the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the medicinal product. Any suspected adverse events should be reported to the Ministry of Health according to the National Regulation by using an online form https://sideeffects.health.gov.il

שימוש לפי פנקס קופ''ח כללית 1994
לא צוין
תאריך הכללה מקורי בסל
לא צוין
הגבלות
לא צוין
מידע נוסף
