Quest for the right Drug
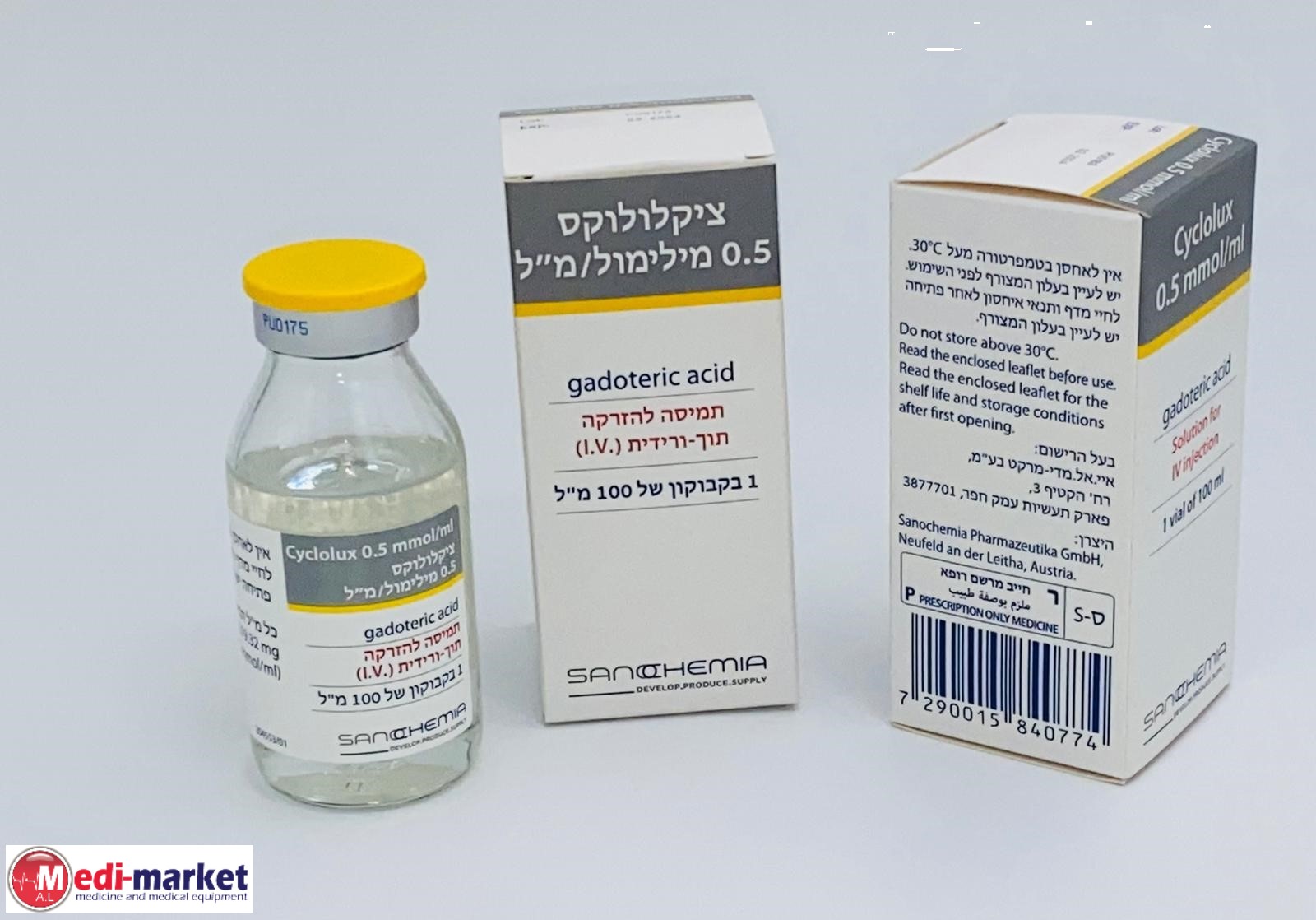
ציקלולוקס 0.5 מילימול / מ"ל CYCLOLUX 0.5 MMOL/ML (GADOTERIC ACID)
תרופה במרשם
תרופה בסל
נרקוטיקה
ציטוטוקסיקה
צורת מתן:
תוך-ורידי : I.V
צורת מינון:
תמיסה להזרקה : SOLUTION FOR INJECTION
עלון לרופא
מינוניםPosology התוויות
Indications תופעות לוואי
Adverse reactions התוויות נגד
Contraindications אינטראקציות
Interactions מינון יתר
Overdose הריון/הנקה
Pregnancy & Lactation אוכלוסיות מיוחדות
Special populations תכונות פרמקולוגיות
Pharmacological properties מידע רוקחי
Pharmaceutical particulars אזהרת שימוש
Special Warning עלון לרופא
Physicians Leaflet
Pharmaceutical particulars : מידע רוקחי
6 PHARMACEUTICAL PARTICULARS 6.1 List of Excipients Meglumine DOTA Water for injection 6.2 Incompatibilities In the absence of compatibility studies, this medicinal product must not be mixed with other medicinal products. 6.3 Shelf life The expiry date of the product is indicated on the packaging materials. 6.4 Special Precautions for Storage Do not store above 30°C. Chemical and physical in-use stability has been demonstrated for 72 hours at room temperature. From a microbiological point of view, the product should be used immediately. If not used immediately, in-use storage times and conditions prior to use are the responsibility of the user and would normally not be longer than 24 hours at 2 to 8°C, unless opening has taken place in controlled and validated aseptic conditions. 6.5 Nature and contents of container 1 and 10 Type II single-use clear glass vial of 20 and 100 ml, sealed with a stopper of bromobutyl rubber and packed in unit carton box. Not all pack sizes may be marketed. 6.6 Special precautions for disposal and other handling Vial: Prepare a syringe and needle. Raise the plastic disk. Puncture the latter with the needle after cleaning the stopper with a cloth soaked in alcohol. Remove the quantity of product necessary for the examination and inject intravenously. The peel-off tracking label on the vials should be stuck onto the patient record to enable accurate recording of the gadolinium contrast agent used. The dose used should also be recorded. If electronic patient records are used, the name of the product, the batch number and the dose should be entered into the patient record. Any unused medicinal product or waste material should be disposed of in accordance with local requirements. 7 MARKETING AUTHORISATION HOLDER A.L. Medi-Market Ltd., 3 Hakatif St., Emek Hefer Industrail Park, 3877701

שימוש לפי פנקס קופ''ח כללית 1994
לא צוין
תאריך הכללה מקורי בסל
לא צוין
הגבלות
לא צוין
מידע נוסף
