Quest for the right Drug
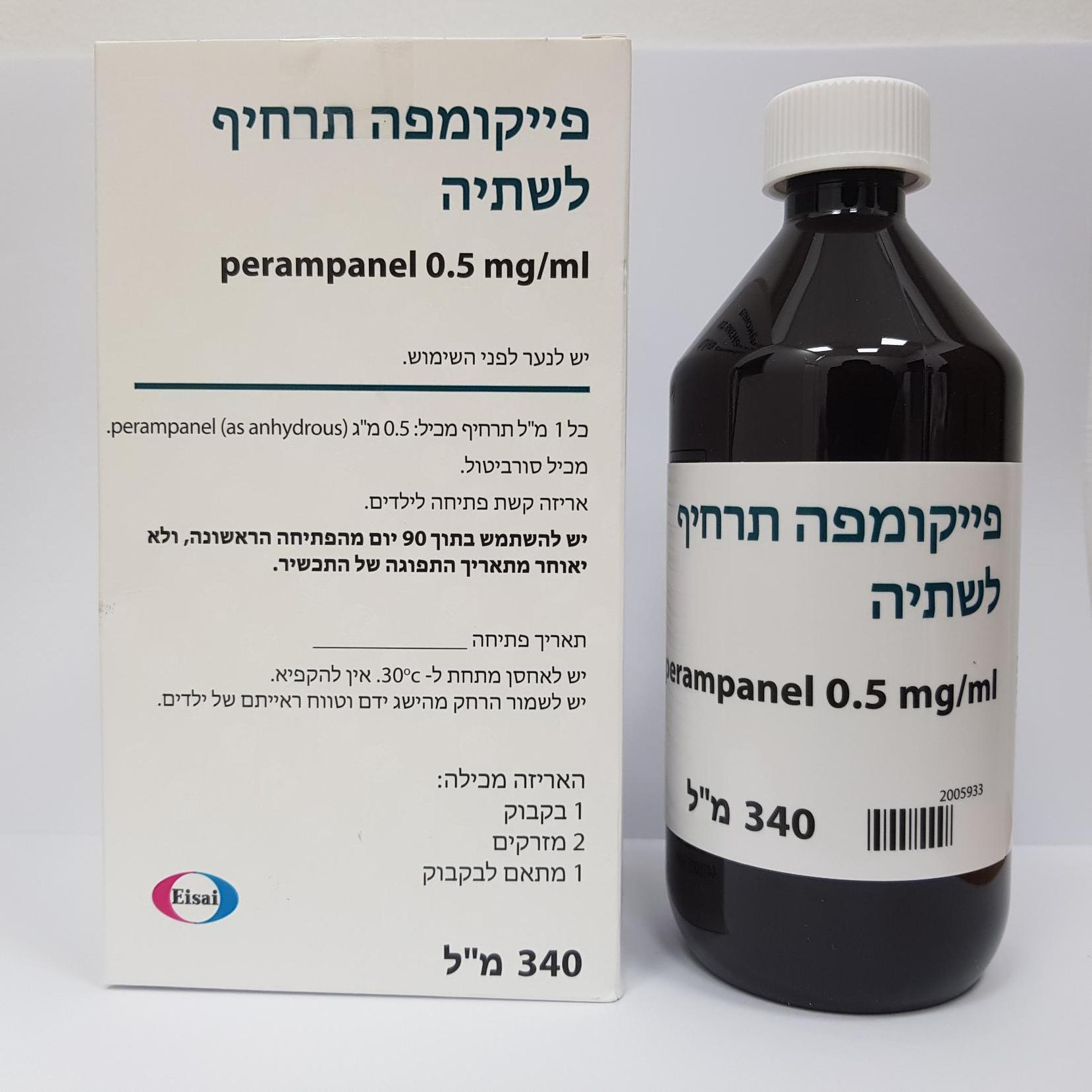
פייקומפה תרחיף לשתיה FYCOMPA ORAL SUSPENSION (PERAMPANEL AS ANHYDROUS)
תרופה במרשם
תרופה בסל
נרקוטיקה
ציטוטוקסיקה
צורת מתן:
פומי : PER OS
צורת מינון:
תרחיף : SUSPENSION
עלון לרופא
מינוניםPosology התוויות
Indications תופעות לוואי
Adverse reactions התוויות נגד
Contraindications אינטראקציות
Interactions מינון יתר
Overdose הריון/הנקה
Pregnancy & Lactation אוכלוסיות מיוחדות
Special populations תכונות פרמקולוגיות
Pharmacological properties מידע רוקחי
Pharmaceutical particulars אזהרת שימוש
Special Warning עלון לרופא
Physicians Leaflet
Adverse reactions : תופעות לוואי
4.8 Undesirable effects Summary of the safety profile In all controlled and uncontrolled trials in patients with partial-onset seizures, 1,639 patients have received perampanel of whom 1,147 have been treated for 6 months and 703 for longer than 12 months. In the controlled and uncontrolled study in patients with primary generalised tonic-clonic seizures, 114 patients have received perampanel of whom 68 have been treated for 6 months and 36 for longer than 12 months. Adverse reactions leading to discontinuation: In the controlled Phase 3 partial-onset seizures clinical trials, the rate of discontinuation as a result of an adverse reaction was 1.7% (3/172), 4.2% (18/431) and 13.7% (35/255) in patients randomised to receive perampanel at the recommended doses of 4 mg, 8 mg and 12 mg/day, respectively, and 1.4% (6/442) in patients randomised to receive placebo. The adverse reactions most commonly (≥1% in the total perampanel group and greater than placebo) leading to discontinuation were dizziness and somnolence. In the controlled Phase 3 primary generalised tonic-clonic seizures clinical trial, the rate of discontinuation as a result of an adverse reaction was 4.9% (4/81) in patients randomized to receive perampanel 8 mg, and 1.2% (1/82) in patients randomized to receive placebo. The adverse reaction most commonly leading to discontinuation (≥2% in the perampanel group and greater than placebo) was dizziness. Post-marketing use Severe cutaneous adverse reactions (SCARs) including drug reaction with eosinophilia and systemic symptoms (DRESS) have been reported in association with perampanel treatment (see section 4.4). Tabulated list of adverse reactions In the table below, adverse reactions, which were identified based on review of the full Fycompa clinical studies safety database, are listed by System Organ Class and frequency. The following convention has been used for the classification of adverse reactions: very common (≥1/10), common (≥1/100 to <1/10), uncommon (≥1/1,000 to <1/100), not known (cannot be estimated from the available data). Within each frequency category, adverse reactions are presented in order of decreasing seriousness. System Organ Very common Common Uncommon Not known Class Metabolism and Decreased System Organ Very common Common Uncommon Not known Class nutrition disorders appetite Increased appetite Psychiatric Aggression Suicidal disorders Anger ideation Anxiety Suicide Confusional attempt state Hallucinations Psychotic disorder Nervous system Dizziness Ataxia disorders Somnolence Dysarthria Balance disorder Irritability Eye disorders Diplopia Vision blurred Ear and labyrinth Vertigo disorders Gastrointestinal Nausea disorders Skin and Drug Reaction subcutaneous with Eosinophilia tissue disorders and Systemic Symptoms (DRESS)* Stevens - Johnson Syndrome (SJS)* Musculoskeletal Back pain and connective tissue disorders General disorders Gait disturbance Fatigue Investigations Weight increased Injury, poisoning Fall and procedural complications * See section 4.4 Paediatric population Based on the clinical trial database of 196 adolescents exposed to perampanel from double- blind studies for partial onset seizures and primary generalized tonic-clonic seizures, the overall safety profile in adolescents was similar to that of adults, except for aggression, which was observed more frequently in adolescents than in adults. Based on the clinical trial database of 180 paediatric patients exposed to perampanel from a multicentre, open label study, the overall safety profile in children was similar to that established for adolescents and adults, except for somnolence, irritability, aggression, and agitation, which were observed more frequently in the paediatric study compared to studies in adolescents and adults. Available data in children did not suggest any clinically significant effects of perampanel on growth and development parameters including body weight, height, thyroid function, insulin-like growth factor-1 (IGF-1) level, cognition (as assessed by Aldenkamp-Baker neuropsychological assessment schedule [ABNAS]), behaviour (as assessed by Child Behavior Checklist [CBCL]), and dexterity (as assessed by Lafayette Grooved Pegboard Test [LGPT]). However, long term effects [greater than 1 year] on learning, intelligence, growth, endocrine function, and puberty in children remain unknown. Reporting of suspected adverse reactions Reporting suspected adverse reactions after authorisation of the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the medicinal product. Any suspected adverse events should be reported to the Ministry of Health according to the National Regulation by using an online form: https://sideeffects.health.gov.il

שימוש לפי פנקס קופ''ח כללית 1994
לא צוין
תאריך הכללה מקורי בסל
לא צוין
הגבלות
לא צוין
מידע נוסף
