Quest for the right Drug
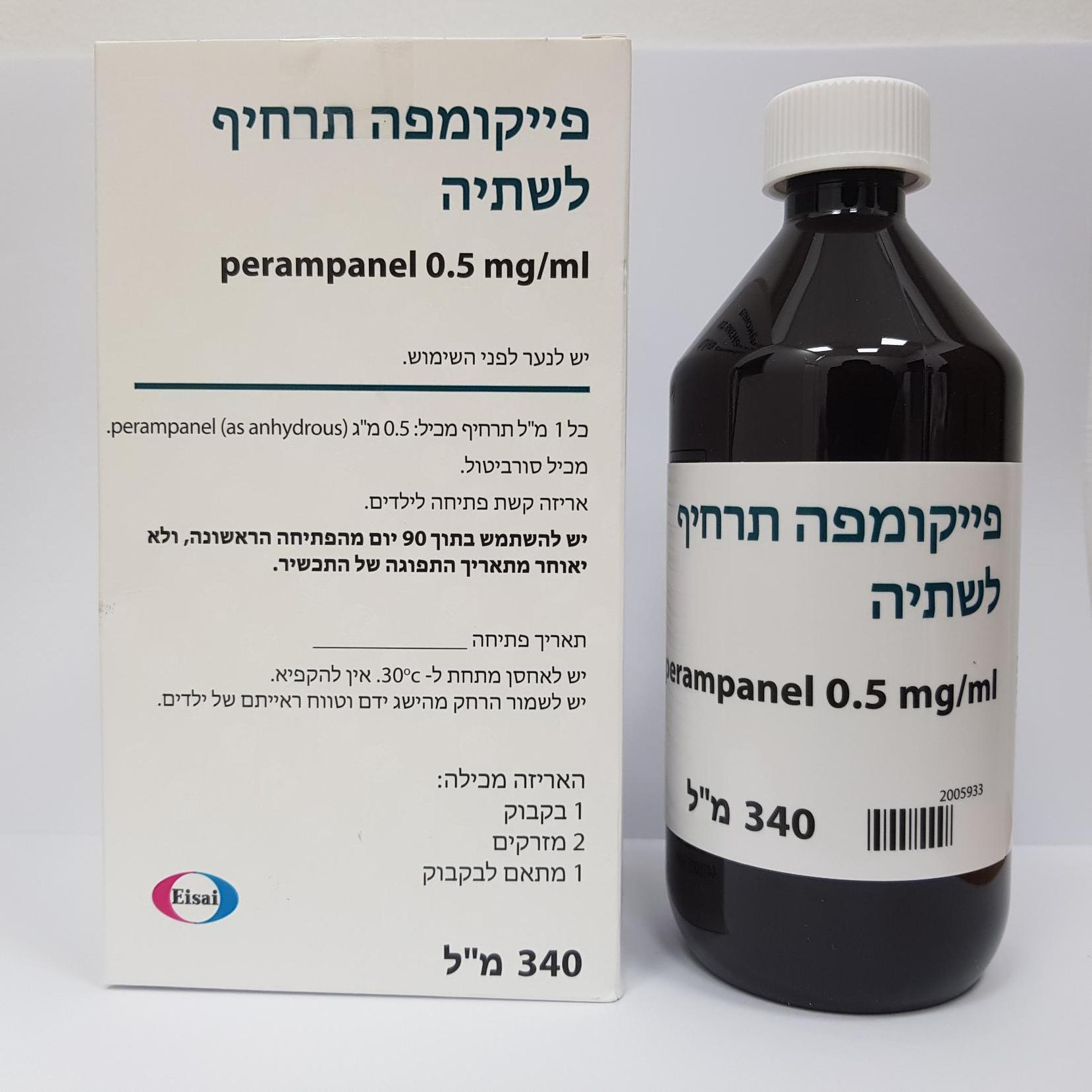
פייקומפה תרחיף לשתיה FYCOMPA ORAL SUSPENSION (PERAMPANEL AS ANHYDROUS)
תרופה במרשם
תרופה בסל
נרקוטיקה
ציטוטוקסיקה
צורת מתן:
פומי : PER OS
צורת מינון:
תרחיף : SUSPENSION
עלון לרופא
מינוניםPosology התוויות
Indications תופעות לוואי
Adverse reactions התוויות נגד
Contraindications אינטראקציות
Interactions מינון יתר
Overdose הריון/הנקה
Pregnancy & Lactation אוכלוסיות מיוחדות
Special populations תכונות פרמקולוגיות
Pharmacological properties מידע רוקחי
Pharmaceutical particulars אזהרת שימוש
Special Warning עלון לרופא
Physicians Leaflet
Pharmaceutical particulars : מידע רוקחי
6. PHARMACEUTICAL PARTICULARS 6.1 List of excipients Fycompa Oral suspension Sorbitol (E420) liquid (crystallising), Microcrystalline cellulose (E460), Carmellose sodium (E466), Citric acid, anhydrous (E330), Sodium benzoate (E211), Poloxamer 188, Simethicone emulsion 30% (containing: purified water, silicone oil, polysorbate 65, methylcellulose, silica gel, macrogol stearate, sorbic acid, benzoic acid (E210) and sulfuric acid) and Purified water. 2 mg tablet Core Lactose monohydrate, Low-substituted hydroxypropyl cellulose, Povidone, Magnesium stearate Film coating Hypromellose 2910; Talc; Macrogol 8000; Titanium dioxide; Ferric oxide, yellow; Ferric oxide, red 4 mg tablet Core Lactose monohydrate, Low-substituted hydroxypropyl cellulose, Povidone , Magnesium stearate Film coating Hypromellose 2910; Talc; Macrogol 8000; Titanium dioxide ; Ferric oxide, red 6 mg tablet Core Lactose monohydrate, Low-substituted hydroxypropyl cellulose, Povidone , Microcrystalline cellulose, Magnesium stearate Film coating Hypromellose 2910; Talc; Macrogol 8000; Titanium dioxide ; Ferric oxide, red 8 mg tablet Core Lactose monohydrate, Low-substituted hydroxypropyl cellulose, Povidone, Microcrystalline cellulose, Magnesium stearate Film coating Hypromellose 2910; Talc; Macrogol 8000; Titanium dioxide; Ferric oxide, red; Ferric oxide, black 10 mg tablet Core Lactose monohydrate, Low-substituted hydroxypropyl cellulose, Povidone, Microcrystalline cellulose, Magnesium stearate Film coating Hypromellose 2910; Talc; Macrogol 8000; Titanium dioxide; Ferric oxide, yellow; FD&C Blue #2 Indigo carmine aluminium lake 12 mg tablet Core Lactose monohydrate, Low-substituted hydroxypropyl cellulose, Povidone, Microcrystalline cellulose, Magnesium stearate Film coating Hypromellose 2910; Talc; Macrogol 8000; Titanium dioxide; FD&C Blue #2 Indigo carmine aluminium lake 6.2 Incompatibilities Not applicable. 6.3 Shelf life The expiry date of the product is indicated on the packaging materials. Fycompa Oral suspension After first opening: Use within 90 days and not later than the expiry date indicated on the packaging materials. 6.4 Special precautions for storage Store below 30°C. Do not freeze. 6.5 Nature and contents of container Fycompa Oral suspension Polyethylene terephthalate (PET) bottle with a child-resistant (CR) polypropylene (PP) closure; each bottle contains 340 ml of suspension in an outer cardboard carton. Each carton contains one bottle, two 20 mL graduated oral dosing syringes and an LDPE press-in bottle adapter (PIBA). The oral dosing syringes are graduated in 0.5 ml increments. Film-coated tablets PVC/aluminium blisters 2 mg – pack of 7, 10, 14, 28, 84, 98 4 mg – packs of 7, 10, 14, 28, 84, 98 6 mg – packs of 7, 10, 14, 28, 84, 98 8 mg – packs of 7, 10, 14, 28, 84, 98 10 mg – packs of 7, 10, 14, 28, 84, 98 12 mg – packs of 7, 10, 14, 28, 84, 98 Not all pack sizes may be marketed. 6.6 Special precautions for disposal No special requirements for disposal. Any unused medicinal product or waste material should be disposed of in accordance with local requirements.

שימוש לפי פנקס קופ''ח כללית 1994
לא צוין
תאריך הכללה מקורי בסל
לא צוין
הגבלות
לא צוין
מידע נוסף
