Quest for the right Drug
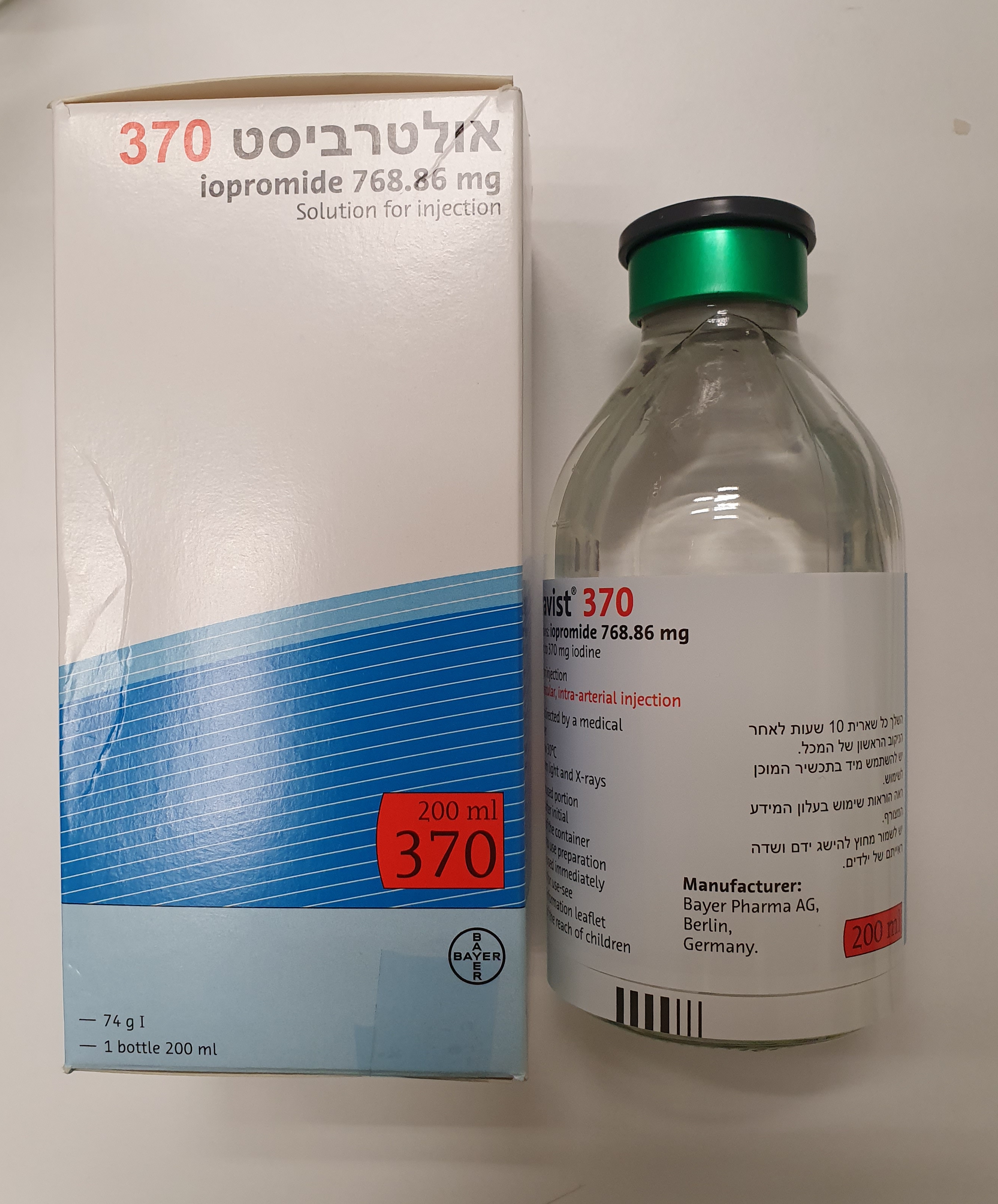
אולטרביסט 370 ULTRAVIST 370 (IOPROMIDE)
תרופה במרשם
תרופה בסל
נרקוטיקה
ציטוטוקסיקה
צורת מתן:
תוך-ורידי, תוך-עורקי : I.V, INTRA-ARTERIAL
צורת מינון:
תמיסה להזרקה : SOLUTION FOR INJECTION
עלון לרופא
מינוניםPosology התוויות
Indications תופעות לוואי
Adverse reactions התוויות נגד
Contraindications אינטראקציות
Interactions מינון יתר
Overdose הריון/הנקה
Pregnancy & Lactation אוכלוסיות מיוחדות
Special populations תכונות פרמקולוגיות
Pharmacological properties מידע רוקחי
Pharmaceutical particulars אזהרת שימוש
Special Warning עלון לרופא
Physicians Leaflet
Adverse reactions : תופעות לוואי
4.8 Undesirable effects 4.8.1 Summary of the safety profile The general safety profile of Ultravist is based on data obtained in pre-marketing studies in more than 3,900 patients and post-marketing studies in more than 74,000 patients, as well as on data from spontaneous reporting and the literature. The most frequently reported undesirable effects (> 4%) in patients administered Ultravist are headaches, nausea and vasodilation. The most serious undesirable effects that occur in patients administered Ultravist are as follows: anaphylactoid shock, respiratory arrest, bronchospasm, laryngeal oedema, pharyngeal oedema, asthma, coma, cerebral infarction, cerebrovascular accident, cerebral oedema, convulsions, arrhythmia, cardiac arrest, myocardial ischaemia, myocardial infarction, heart failure, bradycardia, cyanosis, hypotension, shock, dyspnoea, pulmonary oedema, respiratory failure and aspiration. 4.8.2 Summary table of undesirable effects The undesirable effects observed with Ultravist are summarised in the table below. They are listed by System Organ Class (MedDRA version 13.0), the most appropriate MedDRA term being used to describe a specific reaction with its synonyms and related conditions. Undesirable effects reported during clinical trials are classed according to their frequency, with frequency groups being defined according to the following convention: common (≥ 1/100 to < 1/10), uncommon (≥ 1/1,000 to < 1/100), rare (≥ 1/10,000 to < 1/1,000). The undesirable effects reported in the post-marketing period only, whose frequency could not be estimated, are listed under “unknown”. Table 1: Undesirable effects reported in clinical trials or in the post-marketing period in patients treated with Ultravist System organ Common Uncommon Rare Frequency unknown class Immune system Hypersensitivity/ disorders anaphylactoid reactions (anaphylactoid shock§)*), respiratory arrest§)*), bronchospasm*), laryngeal*)/ pharyngeal*)/facial oedema, tongue oedema§), laryngeal/pharyngeal spasm§), asthma§)*), conjunctivitis§), lacrimation§), sneezing, cough, mucosal oedema, rhinitis§), hoarseness§), throat irritation§, urticaria, pruritus, angioedema) Endocrine Thyrotoxic crisis, thyroid disorders disorders Psychiatric Anxiety disorders Nervous system Dizziness, Vasovagal reactions, Coma*), cerebral disorders headaches, confused state, ischaemia/infarction*), dysgeusia restlessness, cerebrovascular paraesthesia/ accident*), cerebral hypoesthesia, oedemaa)*), convulsions*), somnolence transient loss of cortical visiona), loss of consciousness, agitation, amnesia, tremor, speech disorders, paresis/paralysis, contrast media‑induced encephalopathy Eye disorders Vision disorder/ visual acuity disorder Ear and Hearing disorders labyrinth disorders Cardiac Chest pain/ Arrythmia*) Cardiac arrest*), Myocardial infarction*), disorders discomfort myocardial ischaemia*), heart failure*), palpitations bradycardia*), tachycardia, cyanosis*) System organ Common Uncommon Rare Frequency unknown class Vascular Hypertension, Hypotension*) Shock*), thromboembolic disorders vasodilation eventsa), vasospasma) Respiratory, Dyspnoea*) Pulmonary oedema*), thoracic and respiratory failure*), mediastinal aspiration*) disorders Gastrointestinal Vomiting, Abdominal pain Swallowing disorders, disorders nausea swelling of the salivary glands, diarrhoea Skin and Bullous disorders (e.g. subcutaneous Stevens-Johnson or Lyell tissue disorders syndrome), rash, erythema, hyperhidrosis, acute generalised exanthematous pustulosis, drug reaction with eosinophilia and systemic symptoms Musculoskeletal Compartment syndrome and connective in the case of tissue disorders extravasationa) Renal and Renal disordersa), acute urinary kidney injurya) disorders General Pain, injection Oedema Feeling faint, shivering, disorders and site reactions pallor administration (of various site conditions types such as pain, feeling of warmth§), oedema§), inflammation§) , soft tissue lesions§) in the case of extravasation), hot flushes Investigations Fluctuation in body temperature *) life-threatening and/or fatal cases have been reported a) during intravascular use only §) identified only during post-marketing surveillance (frequency not known) Most reactions following use in body cavities occur a few hours after administration. Reporting of suspected adverse reactions Reporting suspected adverse reactions after authorisation of the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the medicinal product. Any suspected adverse events should be reported to the Ministry of Health according to the National Regulation by using an online form https://sideeffects.health.gov.il

שימוש לפי פנקס קופ''ח כללית 1994
לא צוין
תאריך הכללה מקורי בסל
לא צוין
הגבלות
לא צוין
מידע נוסף
