Quest for the right Drug
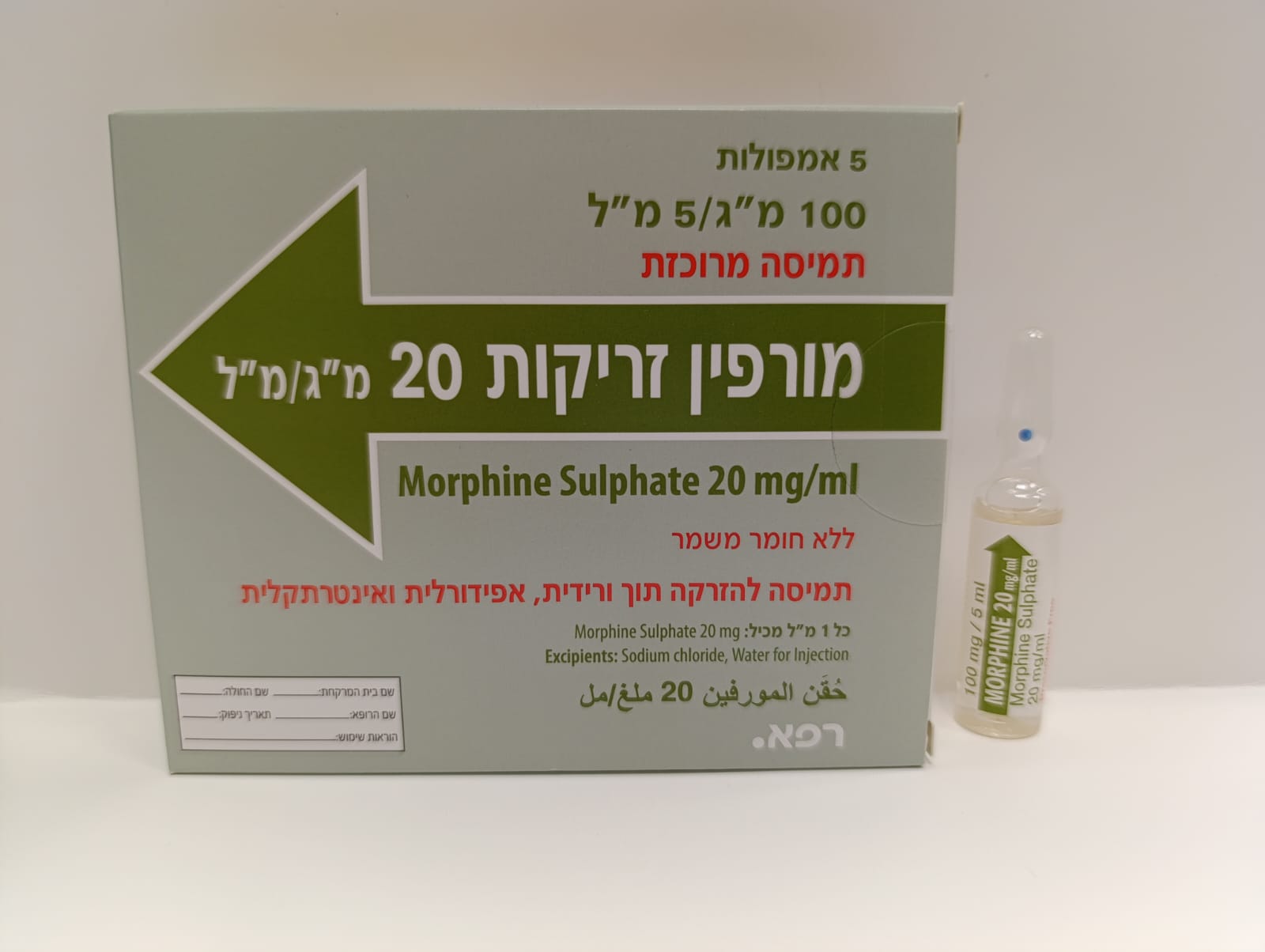
מורפין זריקות 20 מ"ג/מ"ל MORPHINE INJECTIONS 20 MG/ML (MORPHINE SULFATE)
תרופה במרשם
תרופה בסל
נרקוטיקה
ציטוטוקסיקה
צורת מתן:
תוך-שדרתי, תוך-ורידי, אפידורל : INTRATHECAL, I.V, EPIDURAL
צורת מינון:
תמיסה להזרקה : SOLUTION FOR INJECTION
עלון לרופא
מינוניםPosology התוויות
Indications תופעות לוואי
Adverse reactions התוויות נגד
Contraindications אינטראקציות
Interactions מינון יתר
Overdose הריון/הנקה
Pregnancy & Lactation אוכלוסיות מיוחדות
Special populations תכונות פרמקולוגיות
Pharmacological properties מידע רוקחי
Pharmaceutical particulars אזהרת שימוש
Special Warning עלון לרופא
Physicians Leaflet
Overdose : מינון יתר
4.9 OVERDOSAGE Clinical Presentation Acute overdose with MORPHINE INJECTIONS 20 MG/ML can be manifested by respiratory depression, somnolence progressing to stupor or coma, skeletal muscle flaccidity, cold and clammy skin, constricted pupils, and, in some cases, pulmonary edema, bradycardia, hypotension, hypoglycemia, partial or complete airway obstruction, atypical snoring, and death. Marked mydriasis rather than miosis may be seen with hypoxia in overdose situations [see Pharmaceutical properties (5)]. Treatment of Overdose In case of overdose, priorities are the reestablishment of a patent and protected airway and institution of assisted or controlled ventilation, if needed. Employ other supportive measures (including oxygen and vasopressors) in the management of circulatory shock and pulmonary edema as indicated. Cardiac arrest or arrhythmias will require advanced life-support measures. Opioid antagonists, such as naloxone , are specific antidotes to respiratory depression resulting from opioid overdose. For clinically significant respiratory or circulatory depression secondary to morphine overdose, administer an opioid antagonist. Neuraxial: As the duration of effect of naloxone is considerably shorter than that of epidural or intrathecal morphine, repeated administration may be necessary. Patients should be closely observed for evidence of renarcotization. Because the duration of opioid reversal is expected to be less than the duration of action of morphine in MORPHINE INJECTIONS 20 MG/ML, particularly with epidural or intrathecal morphine, carefully monitor the patient until spontaneous respiration is reliably re-established. If the response to an opioid antagonist is suboptimal or only brief in nature, administer additional antagonist as directed by the product’s prescribing information. In an individual physically dependent on opioids, administration of the recommended usual dosage of the antagonist will precipitate an acute withdrawal syndrome. The severity of the withdrawal symptoms experienced will depend on the degree of physical dependence and the dose of the antagonist administered. If a decision is made to treat serious respiratory depression in the physically-dependent patient, administration of the antagonist should be begun with care and by titration with smaller than usual doses of the antagonist.

מסגרת הכללה בסל
התוויות הכלולות במסגרת הסל
| התוויה | תאריך הכללה | תחום קליני | Class Effect | מצב מחלה |
|---|---|---|---|---|
| MORPHINE | ||||
| HYDROMORPHONE | ||||
| For the relief of severe pain in cancer. |
שימוש לפי פנקס קופ''ח כללית 1994
Severe and intractable oncological and postoperative pain
תאריך הכללה מקורי בסל
01/01/1995
הגבלות
תרופה שאושרה לשימוש כללי בקופ'ח
מידע נוסף
