Quest for the right Drug
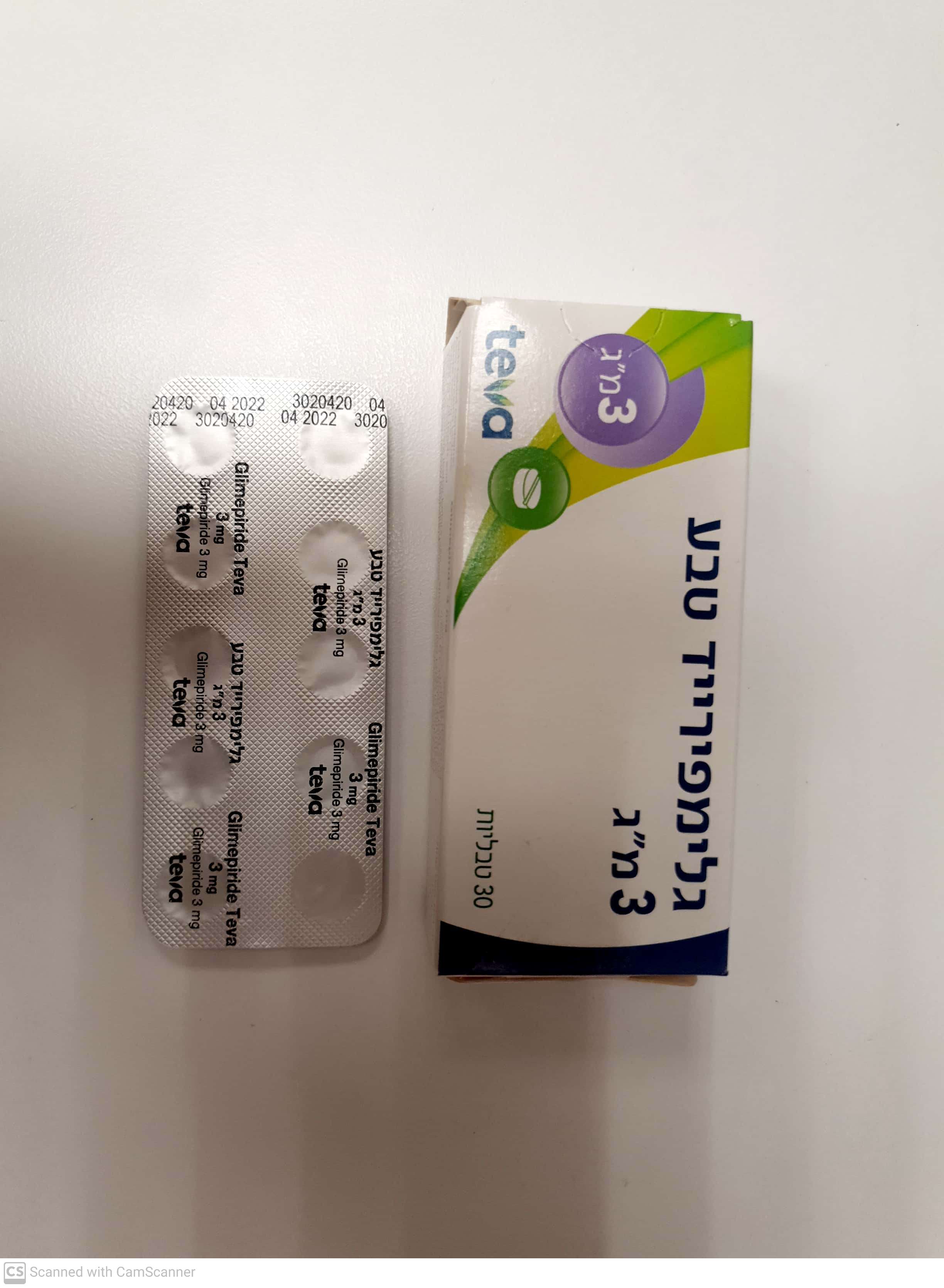
גלימפירייד טבע ® 3 מ"ג GLIMEPIRIDE TEVA ® 3 MG (GLIMEPIRIDE)
תרופה במרשם
תרופה בסל
נרקוטיקה
ציטוטוקסיקה
צורת מתן:
פומי : PER OS
צורת מינון:
טבליה : TABLETS
עלון לרופא
מינוניםPosology התוויות
Indications תופעות לוואי
Adverse reactions התוויות נגד
Contraindications אינטראקציות
Interactions מינון יתר
Overdose הריון/הנקה
Pregnancy & Lactation אוכלוסיות מיוחדות
Special populations תכונות פרמקולוגיות
Pharmacological properties מידע רוקחי
Pharmaceutical particulars אזהרת שימוש
Special Warning עלון לרופא
Physicians Leaflet
Adverse reactions : תופעות לוואי
4.8 Undesirable effects The following adverse reactions from clinical investigations were based on experience with glimepiride and other sulfonylureas, were listed below by system organ class and in order of decreasing incidence: very common (≥ 1/10), common (≥ 1/100 to < 1/10), uncommon (≥ 1/1,000 to < 1/100), rare (≥ 1/10,000 to < 1/1,000), very rare (< 1/10,000), not known (cannot be estimated from the available data). Blood and lymphatic system disorders Rare: thrombocytopenia, leukopenia, granulocytopenia, agranulocytosis, erythropenia, hemolytic anemia and pancytopenia, which are in general reversible upon discontinuation of medication. Not known: severe thrombocytopenia with platelet count less than 10,000/μl and thrombocytopenic purpura. Immune system disorders Very rare: leukocytoclastic vasculitis, mild hypersensitivity reactions that may develop into serious reactions with dyspnea, fall in blood pressure and sometimes shock. Not known: cross-allergenicity with sulfonylureas, sulfonamides or related substances is possible. Metabolism and nutrition disorders Rare: hypoglycemia These hypoglycemic reactions mostly occur immediately, may be severe and are not always easy to correct. The occurrence of such reactions depends, as with other hypoglycemic therapies, on individual factors such as dietary habits and dose (see further under section 4.4). Eye disorders Not known: visual disturbances, transient, may occur especially on initiation of treatment, due to changes in blood glucose levels. Gastrointestinal disorders Rare: dysgeusia. Very rare: nausea, vomiting, diarrhea, abdominal distension, abdominal discomfort and abdominal pain, which seldom lead to discontinuation of therapy. Hepatobiliary disorders Very rare: hepatic function abnormal (e.g., with cholestasis and jaundice), hepatitis and hepatic failure. Not known: hepatic enzymes increased. Skin and subcutaneous tissue disorders Rare: alopecia. Not known: hypersensitivity reactions of the skin may occur such as pruritus, rash, urticaria and photosensitivity. Investigations Rare: weight gain. Very rare: blood sodium decrease. Reporting of suspected adverse reactions Reporting suspected adverse reactions after authorisation of the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the medicinal product. Any suspected adverse events should be reported to the Ministry of Health according to the National Regulation by using an online form: https://sideeffects.health.gov.il.

פרטי מסגרת הכללה בסל
התרופה תינתן לטיפול בסוכרת סוג 2.
מסגרת הכללה בסל
התוויות הכלולות במסגרת הסל
| התוויה | תאריך הכללה | תחום קליני | Class Effect | מצב מחלה |
|---|---|---|---|---|
| התרופה תינתן לטיפול בסוכרת סוג 2. |
שימוש לפי פנקס קופ''ח כללית 1994
לא צוין
תאריך הכללה מקורי בסל
15/01/2015
הגבלות
תרופה מוגבלת לרישום ע'י רופא מומחה או הגבלה אחרת
מידע נוסף
