Quest for the right Drug
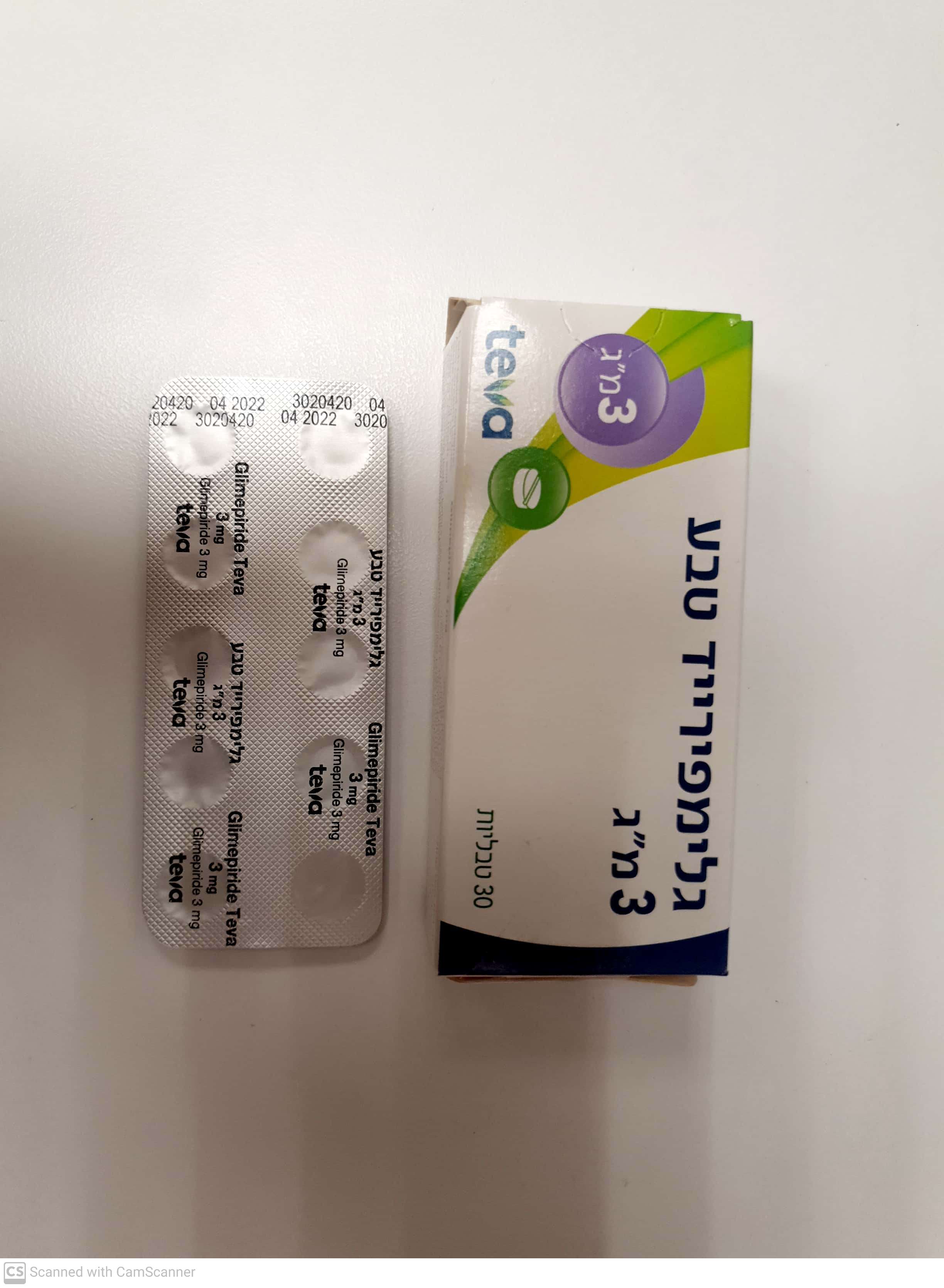
גלימפירייד טבע ® 3 מ"ג GLIMEPIRIDE TEVA ® 3 MG (GLIMEPIRIDE)
תרופה במרשם
תרופה בסל
נרקוטיקה
ציטוטוקסיקה
צורת מתן:
פומי : PER OS
צורת מינון:
טבליה : TABLETS
עלון לרופא
מינוניםPosology התוויות
Indications תופעות לוואי
Adverse reactions התוויות נגד
Contraindications אינטראקציות
Interactions מינון יתר
Overdose הריון/הנקה
Pregnancy & Lactation אוכלוסיות מיוחדות
Special populations תכונות פרמקולוגיות
Pharmacological properties מידע רוקחי
Pharmaceutical particulars אזהרת שימוש
Special Warning עלון לרופא
Physicians Leaflet
Overdose : מינון יתר
4.9 Overdose Symptoms After ingestion of an overdose hypoglycemia may occur, lasting from 12 to 72 hours, and may recur after an initial recovery. Symptoms may not be present for up to 24 hours after ingestion. In general observation in hospital is recommended. Nausea, vomiting and epigastric pain may occur. The hypoglycemia may in general be accompanied by neurological symptoms like restlessness, tremor, visual disturbances, co-ordination problems, sleepiness, coma and convulsions. Acute overdosage as well as long term treatment with too high a dose of glimepiride may lead to severe life-threatening hypoglycemia. Management As soon as an overdose of glimepiride has been discovered, a physician must be notified without delay. The patient must immediately take sugar, if possible, in the form of glucose, unless a physician has already undertaken responsibility for treating the overdose. Careful monitoring is essential until the physician is confident that the patient is out of danger. It must be remembered that hypoglycemia may recur after initial recovery. In case of mild episode of hypoglycemia, treatment primarily consists of oral glucose. Severe hypoglycemic reactions requires immediate treatment. Significant overdoses of glimepiride and severe reactions with signs such as loss of consciousness or other serious neurological disorders are medical emergencies and require immediate treatment. Admission to hospital in an intensive care department is indicated. If large quantities of glimepiride have been ingested, gastric lavage is indicated within 1 hour of ingestion, followed by activated charcoal, sodium-sulphate and octreotide. Start the administration of glucose as soon as possible, if necessary by a bolus intravenous injection of 50 ml of a 50% solution, followed by an infusion of a 10% solution with strict monitoring of blood glucose for at least 24 hours. Alternatively, in adults, administration of glucagon may be considered. Further treatment should be symptomatic. In severe cases with a protracted course, hypoglycemia, or the danger of slipping back into hypoglycemia, may persist for several days. Pediatric population In particular when treating hypoglycemia due to accidental intake of glimepiride in infants and young children, the dose of glucose given must be carefully controlled to avoid the possibility of producing dangerous hyperglycemia. Blood glucose should be closely monitored.

פרטי מסגרת הכללה בסל
התרופה תינתן לטיפול בסוכרת סוג 2.
מסגרת הכללה בסל
התוויות הכלולות במסגרת הסל
| התוויה | תאריך הכללה | תחום קליני | Class Effect | מצב מחלה |
|---|---|---|---|---|
| התרופה תינתן לטיפול בסוכרת סוג 2. |
שימוש לפי פנקס קופ''ח כללית 1994
לא צוין
תאריך הכללה מקורי בסל
15/01/2015
הגבלות
תרופה מוגבלת לרישום ע'י רופא מומחה או הגבלה אחרת
מידע נוסף
